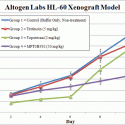
Altogen Labs is a GLP-compliant global contract research organization (CRO) providing comprehensive preclinical research services to pharmaceutical companies, biotechnology firms, and cancer research institutions worldwide. Headquartered in Austin, Texas, Altogen Labs supports the development of new therapeutics by offering cutting-edge laboratory capabilities in oncology, toxicology, gene expression, and pharmacology. With a focus on accelerating the discovery of novel anti-cancer treatments, Altogen Labs has established an extensive portfolio of over 100 in-house validated xenograft models and advanced in vivo toxicology services for investigational new drug (IND) studies.
The company’s oncology research services include conventional cell line-derived xenografts (CDX), patient-derived xenografts (PDX), patient-derived cell cultures (PDC), and organoids (PDOrg), enabling accurate modeling of human cancer biology and therapeutic responses. These models are genetically and clinically characterized to improve translational relevance and predict drug efficacy with high confidence. Altogen Labs also offers advanced immuno-oncology platforms utilizing humanized and immunodeficient rodent models, including those engrafted with PBMCs, CD34+ hematopoietic stem cells, and iPSCs. These platforms facilitate the investigation of tumor-immune system interactions and immunotherapeutic strategies.
Efficacy and safety studies are critical components of preclinical drug development. Efficacy studies evaluate whether a compound produces its intended therapeutic effect under controlled conditions, optimize dosing strategies, and determine treatment duration and administration routes. In parallel, safety studies assess the toxicological profile of investigational drugs, including acute, sub-chronic, and chronic toxicity, as well as their effects on major organ systems. Altogen Labs offers both efficacy and safety services designed to meet regulatory requirements and support successful IND applications.
In addition to oncology, Altogen Labs conducts preclinical studies in fields such as inflammation, immunology, neurodegenerative disease, pain, and metabolic disorders. Core services include xenograft tumor growth delay (TGD) studies, in vivo toxicology, RNAi and gene expression analysis, biodistribution and imaging studies, stable cell line development, and tissue culture testing. These integrated services ensure a thorough evaluation of a compound’s pharmacological activity, safety, and therapeutic potential.
By combining scientific expertise with cutting-edge technology, Altogen Labs serves as a trusted research partner dedicated to accelerating the translation of laboratory discoveries into clinical therapies.
Contact Information:
Altogen Labs | 11200 Menchaca Rd | Austin, TX 78748 USA
Tel: 512-433-6177 | Web: altogenlabs.com