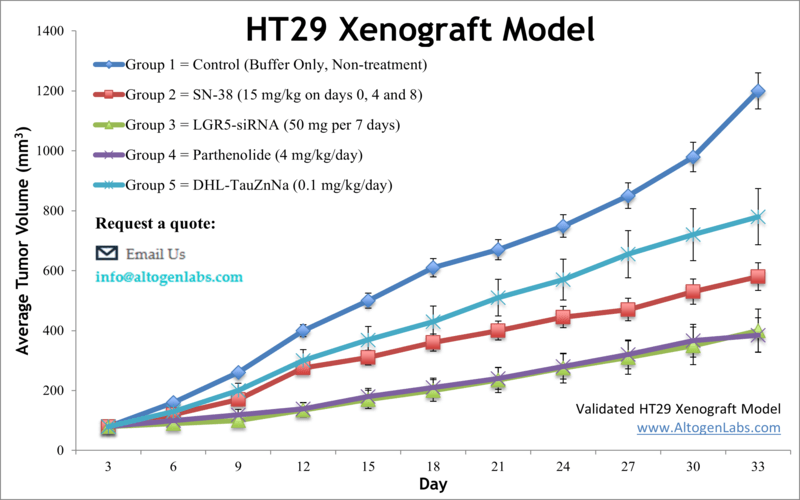
HT29 xenograft model (subcutaneous and metastatic)
HT-29 is a human colon cancer cell line that was originally derived from a primary adenocarcinoma tumor in a patient with colon cancer. It is widely used in cancer research as a model system to study various aspects of cancer biology, including cell proliferation, differentiation, and drug resistance. Colon cancer represents the third most prevalent malignancy among both males and females and the second primary cause of cancer-related deaths in the United States. HT29 is a human colorectal adenocarcinoma cell line that was derived from a tumor of a 44-year-old Caucasian woman in 1964 and is helpful in gaining insight into the etiology of human colon cancers. The HT29 colorectal cell line is vital for examining biochemical pathways that aid in the development of colon carcinoma. Many colorectal rodent models have been developed to facilitate studies of human malignancies, allowing a better understanding of mechanisms contributing to tumor growth. Example studies using the HT29 model include the Cancer Research article by Radulovic et al. in which the group identified that RC-3095, a peptide agonist designed to release bombesin and gastrin, successfully inhibited tumor growth in HT29 xenografts. This study contributed to knowledge regarding involvement of sex steroids, hormones and growth factors on colorectal tumorigenesis. HT29 was featured in a model spotlight in 2016 (Maryland Franklin, PhD, MI Bioesearch) due to its high utilization and success in helping identify chemotherapy treatments. A 2016 Nature study (Teng et al.) used HT29 as a CRC model to demonstrate the oncogenic role of protein tyrosine phosphatase 1B (PTP1B); results confirmed that PTP1b predicts poor clinical outcomes in patients, identified PITX1 as a novel substrate on the p120RasGAP axis and found that the drug regorafenab has an antitumor effect that is mediated by PTP1B. This study established a role of PTP1B in colorectal cancer and demonstrated its potential as a biomarker for predicting the effectiveness of regorafenib. Finally, a 2014 study in Oncoscience (Gotnik et al.) used the HT29 model to study the mechanism of resistance of colorectal cancer against sunitinib, a tyrosine kinase and angiogenesis inhibitor. Often tumor resistance can be attributed to microenvironmental host-factors such as tumor microvasculature or biodistribution however results showed that resistant tumor cells demonstrated increased sequestration in cells via lysosomes. Therefore the study concluded to overcome sinitinib resistance, combination therapy with a treatment that inhibits lysosomal function may be beneficial. The HT29 cell line is used to create the CDX (Cell Line Derived Xenograft) HT29 xenograft mouse model. The HT29 xenograft model enables analysis of the anti-tumor efficacy of EGFR inhibitors (e.g. cetuximab) and to elucidate the mechanism of acquired resistance to tyrosine kinase inhibitors (TKIs), such as using a sunitinib-resistant HT29 cell line.
Download Altogen Labs HT29 Xenograft Model PowerPoint Presentation: ![]()
Basic study design
- Prior to collecting the cells, exponential growth is maintained.
- All cells are collected by trypsinizing the cells in flasks and combining all cell suspensions. Cell count and viability is properly determined using trypan blue (min 98% viability). Cell suspensions are adjusted to an appropriate density for inoculation.
- One million cells of the Matrigel plus HT29 cell suspension (vol=100 µL) is injected s.c. into the flank of one hind leg of each mouse (10 to 12 weeks; NOD/SCID or athymic BALB/C).
- All injection sites are monitored until tumors (palpated) are established. Calipers (digital) are used to measure the tumors pending average sizes of 80-120 mm3.
- After sorting (randomization), the test materials are administered according to the client supplied treatment schedule.
- Daily measurements of the tumor are logged and mouse weights are recorded (up to 3 times weekly).
- The animals are euthanized humanely as the study’s maximum tumor size is reached (or 2,000 mm3).
- A necropsy is performed to remove the tumors, record their weight and then document with digital imaging.
- Tissues are collected as discussed and are stabilized in RNAlater, snap frozen, nucleic acids isolated or tissues are prepared for histological analysis.
Metastatic Model
CDX models are mouse xenografts used in pre-clinical therapeutic studies. However, as primary tumors proliferate they invade surrounding tissue, become circulatory, survive in circulation, implant in foreign parenchyma and proliferate in the distant tissue. This result leads to an extremely high percentage of death in cancer patients due to metastasis. Metastatic tumor mouse models are utilized to develop novel therapeutic agents that target metastasis (anti-metastatic therapeutics).
To create a metastatic model, the cell line of interest is transfected with vectors containing green fluorescent protein (GFP) or luciferase. Maintained under antibiotic selection, only cells containing the integrated vector will survive. The new cell line clones are capable of stably expressing the gene of interest and are used in metastatic mouse model studies. Although each new cell line clone may contain its own inherent difficulties, the new cell line contains the ability to track internal tumor progression via bioluminescence (luciferase fluorescence after injecting luciferin) or fluorescence (GFP). Internal orthotopic and metastatic tumor growth (not palpable) can now be measured throughout the study, enabling a researcher to gain more insight and additional data in contrast to relying on end of study tumor weight measurements.
Case Study: U87-luc Xenograft Model
An example of Altogen Labs utilizing a luciferase expressing cell line to monitor orthotopic tumor growth is exhibited below. The same ideology of tumor observation is incorporated in metastatic tumor models.
Luciferase expressing U87-luc cells were implanted and tumors allowed to grow. Tumor growth was monitored in a Night Owl (Berthold Technologies) imaging system 10 minutes after an intraperitoneal (IP) injection of the luciferin substrate. As seen in the example below, luciferase expression (measured as photons emitted) in the U87-luc model grants the researcher a visual image and quantifiable metric for orthotopic or metastatic tumor progression.
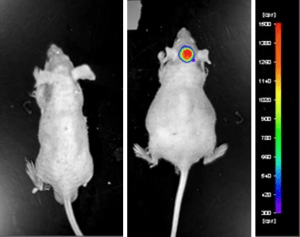
Figure 1. Luciferase expression in U87-luc orthotopic model. Control and implanted glioma mouse model fluorescence was analyzed 10 minutes after intraperitoneal luciferin injection.
View full details of the case study by clicking here.
Get Instant Quote for
HT29 Xenograft Model
Altogen Labs provides an array of laboratory services using over 90 standard Cell Line Derived Xenograft (CDX) models and over 30 PDX models. Following options are available for the HT29 xenograft model:
- HT29 Tumor Growth Delay (TGD; latency)
- HT29 Tumor Growth Inhibition (TGI)
- Dosing frequency and duration of dose administration
- Dosing route (intravenous, intratracheal, continuous infusion, intraperitoneal, intratumoral, oral gavage, topical, intramuscular, subcutaneous, intranasal, using cutting-edge micro-injection techniques and pump-controlled IV injection)
- HT29 tumor immunohistochemistry
- Alternative cell engraftment sites (orthotopic transplantation, tail vein injection and left ventricular injection for metastasis studies, injection into the mammary fat pad, intraperitoneal injection)
- Blood chemistry analysis
- Toxicity and survival (optional: performing a broad health observation program)
- Gross necropsies and histopathology
- Positive control group employing cyclophosphamide, at a dosage of 50 mg/kg administered by intramuscular injection to the control group daily for the study duration
- Lipid distribution and metabolic assays
