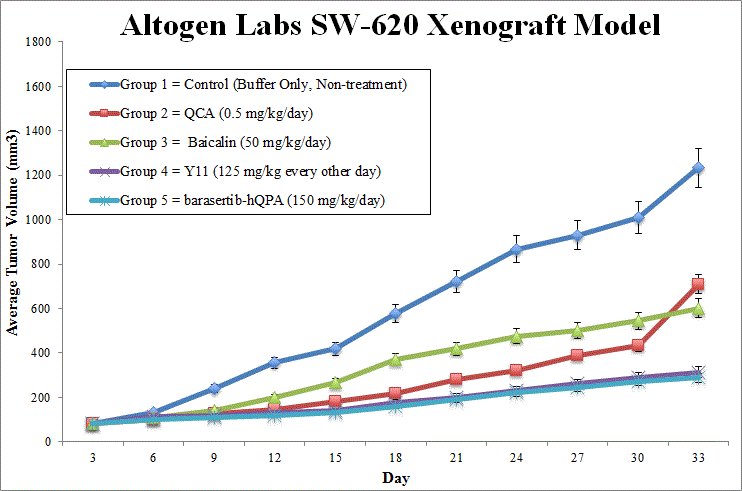
Validated SW-620 xenograft model
The SW620 cell line is a human colorectal adenocarcinoma model originally derived from a metastatic lymph node lesion in a 51-year-old male patient. As a tumorigenic epithelial line established from a secondary tumor of the same individual who provided the SW480 primary tumor line, SW620 offers a powerful system for comparative studies on cancer progression, metastasis, and treatment resistance. This pairing uniquely enables researchers to examine molecular and phenotypic differences between early-stage and metastatic colorectal cancers within a genetically related context. SW620 cells exhibit a fibroblast-like morphology and are distinguished by their enhanced tumorigenicity, metastatic potential, and resistance to apoptosis induced by tumor necrosis factor-alpha (TNF-α) and Fas signaling, characteristics consistent with advanced disease stage. Molecularly, SW620 cells express carcinoembryonic antigen (CEA), transforming growth factor-alpha (TGF-α), and matrilysin, a matrix metalloproteinase implicated in extracellular matrix remodeling. They are negative for colon-specific antigen (CSAp) and colon antigen 3, yet stain positive for keratin via immunoperoxidase techniques. These markers contribute to their utility as a well-characterized model for studying epithelial-to-mesenchymal transition, immune evasion, and invasive behavior in colorectal cancer. Early studies demonstrated the suitability of SW620 xenografts for high-throughput in vivo screening of anticancer agents, with tumor growth kinetics closely matching those observed in clinical colorectal malignancies. Histologically, SW620-derived tumors form poorly differentiated solid sheets with necrotic cores and minimal spontaneous metastasis, which enables consistent experimental reproducibility in preclinical drug evaluation. SW620 xenografts have been utilized in diverse therapeutic investigations, including early work that validated their capacity for identifying candidate chemotherapeutic compounds. More recently, oncolytic virotherapy studies using the replication-competent vaccinia virus GLV-1h68 demonstrated significant tumor growth inhibition and increased survival in treated animals. Mechanistic analyses revealed enhanced expression of immune-modulating cytokines such as IP-10, IFN-γ, MCP-1, and RANTES, alongside notable infiltration of tumor-associated macrophages and natural killer (NK) cells, supporting the immunostimulatory potential of this virotherapy platform. Furthermore, SW620 has proven instrumental in the evaluation of small molecule inhibitors targeting Rho GTPase signaling pathways, such as AZA197, which affect cytoskeletal organization, cell motility, and metastatic competence. Altogen Labs utilizes the SW620 cell line to generate robust cell line-derived xenograft (CDX) models in immunodeficient mice for studying advanced colorectal cancer. These models are particularly valuable in the preclinical assessment of agents directed at late-stage disease, including immunotherapies, apoptosis-modulating drugs, and inhibitors of metastatic signaling cascades. The reproducible tumor growth kinetics, well-characterized molecular signature, and clinical relevance of the SW620 xenograft model make it a critical component of translational research programs aimed at improving outcomes for patients with metastatic colorectal carcinoma.
SW620 Colon Cancer CRC Subcutaneous, Orthotopic And Metastatic Xenograft Models ![]()
Download Altogen Labs SW620 Xenograft Model PowerPoint Presentation: ![]()
SW620 Orthotopic Model for Metastatic CRC Research
Orthotopic xenograft transplantation is a powerful approach for modeling colorectal cancer with enhanced biological relevance, as it permits tumor establishment within the native anatomical site. In contrast to subcutaneous models, orthotopic implantation into the colon or rectum replicates critical aspects of the tumor microenvironment, including tissue architecture, stromal composition, and patterns of invasion. The SW620 cell line, derived from a metastatic colorectal adenocarcinoma, is particularly well-suited for orthotopic transplantation due to its aggressive phenotype and intrinsic capacity for local invasion and distant dissemination. This model supports the evaluation of tumor growth, epithelial-to-mesenchymal transition, angiogenesis, and metastatic potential in a setting that closely mirrors the clinical behavior of advanced colorectal cancer.
When introduced into the colonic or rectal wall of immunocompromised mice, SW620 cells consistently form tumors that demonstrate features of poorly differentiated adenocarcinoma, including disorganized glandular structures, stromal remodeling, and increased neovascularization. The orthotopic setting promotes interactions with surrounding tissue that influence tumor progression and response to therapy, offering a refined platform for mechanistic studies. Our investigations using this model have revealed that non-coding RNAs play a pivotal role in modulating epithelial plasticity, immune escape, and metastatic efficiency.
Modeling Colorectal Metastasis with SW620 Xenografts
Metastatic xenograft transplantation provides a powerful in vivo approach for investigating the biological mechanisms that underlie cancer dissemination and colonization at distant organ sites. These models are particularly valuable for replicating the complex, multi-step processes of metastasis, including intravasation, survival in circulation, extravasation, and secondary tumor formation. The SW620 cell line, derived from a lymph node metastasis of colorectal adenocarcinoma, serves as an ideal system for modeling these phenomena due to its inherently aggressive phenotype, high migratory capacity, and resistance to apoptosis during cellular detachment. Its mesenchymal gene expression profile and ability to survive under hypoxic and anchorage-independent conditions further enhance its utility in studying metastatic behavior.
Metastatic transplantation using SW620 can be performed through several injection routes, such as tail vein, intrasplenic, or orthotopic inoculation, depending on the desired site of metastatic colonization. These methods lead to the development of secondary tumors in organs such as the liver, lungs, and lymph nodes, reflecting patterns observed in advanced colorectal cancer. Resulting metastases exhibit hallmark features of invasive disease, including disorganized tissue architecture, stromal remodeling, and increased vascularization. Experimental use of this model supports the investigation of molecular drivers of metastatic progression, including signaling pathways involved in epithelial-to-mesenchymal transition, survival under stress, and immune evasion. Integration of transcriptomic and functional analyses in this context enables identification of candidate regulators that contribute to systemic dissemination and therapeutic resistance. The SW620 metastatic xenograft model thus offers a robust, clinically relevant platform for elucidating the biological underpinnings of metastasis and advancing preclinical evaluation of anti-metastatic therapies.
Oncogenic Drivers of Metastasis in SW620 Cells
SW620 is a metastatic colorectal cancer cell line characterized by aggressive behavior and dysregulated oncogenic signaling. This line exhibits overexpression of key oncogenes such as c-Met, KRAS, and c-Myc, which contribute to uncontrolled cell proliferation, survival, and invasion. Elevated c-Met expression enhances cellular motility and resistance to apoptosis, while mutations in KRAS sustain activation of downstream pathways including PI3K/AKT and MAPK/ERK, independent of upstream receptor input. The transcription factor c-Myc is also markedly upregulated, promoting increased biosynthetic activity and metabolic reprogramming in favor of rapid cell division. Together, these oncogenic drivers establish a signaling environment that supports malignant progression in SW620 cells.
Additional molecular features of SW620 reinforce its metastatic potential. There is consistent upregulation of cell cycle regulators such as cyclin D1 and CDK4, which facilitate unchecked cell cycle progression. Markers of epithelial-to-mesenchymal transition are also dysregulated, with high vimentin expression and diminished E-cadherin levels, indicating enhanced invasiveness. These coordinated molecular alterations suggest a transitionally reprogrammed phenotype optimized for tumor dissemination. The study’s use of gene expression profiling and protein-level assays allows for a comprehensive understanding of transcriptional and post-translational changes, although more granular methods like single-cell sequencing could further elucidate cellular heterogeneity. The oncogene profile of SW620 provides valuable insight into the mechanisms driving metastatic colorectal cancer and highlights potential targets for combinatorial therapy. Future research should investigate the functional interplay among these oncogenes and identify context-specific vulnerabilities for therapeutic intervention.
SW620 Colon Cancer CRC Subcutaneous, Orthotopic And Metastatic Xenograft Models ![]()
Basic study design
- SW-620 cells are continually maintained under the condition of exponential phase growth. Trypsinization cell viability counts are determined via trypan blue exclusion. Total cell suspension concentration is adjusted such that a 0.1-0.2 mL injection of the matrigel + SW620 suspension results in a total of 1 x 10e6 cells inoculated in the hing leg flank of each mouse (athymic BALB/c (Nu/Nu), 10 weeks).
- Injection sites are palpated up to tri-weekly. Upon tumor formation, measurements with digital calipers are made until tumors reach averages 100-150 mm3.
- Animal sorting is randomized and treatment cohorts are formed. Treatment with the compound of interest follows the dosing schedule. While on-study, tumors are calipered daily and body weights recorded.
- Animals are humanely euthanized as tumor size reaches the maximum size of 2,000 cubic millimeters or 35 days post xenotransplantation. Necropsies and tissue collection are performed according to the experimental setup.
- Each tumor is weighed and documented via imaging. Required tissues are snap frozen or stabilized in RNAlater, prepared for histology via 10% NBF or nucleic acids isolated.
Get Instant Quote for
SW620 Xenograft Model
Xenograft animal models are used to assess the effectiveness of drugs against specific types of cancer. New medicines are tested on staged tumor growths that have been engrafted via subcutaneous or orthotopic inoculation in an immunocompromised mouse or rat model. All clinically approved anti-cancer agents have been evaluated with conventional preclinical in vivo models. Xenograft studies can be highly complex, starting with the selection of the appropriate animal model, choice of tumorigenic cell line, administration method, dosing, analysis of tumor growth rates and tumor analysis (histology, mRNA and protein expression levels).
Following options are available for the SW620 xenograft model:
- SW620 Tumor Growth Delay (TGD; latency)
- SW620 Tumor Growth Inhibition (TGI)
- Dosing frequency and duration of dose administration
- Dosing route
- SW620 tumor immunohistochemistry
- Alternative cell engraftment sites (orthotopic transplantation, tail vein injection and left ventricular injection for metastasis studies)
- Blood chemistry analysis
- Toxicity and survival
- Gross necropsies and histopathology
