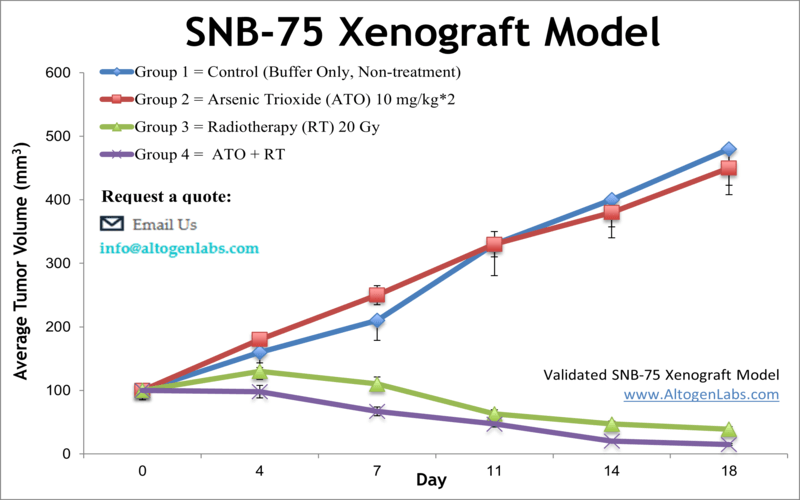
SNB-75 xenograft model
SNB-75 cell line is a human brain tumor cell line. It is also known as SNB-75M. Glioblastoma is the most common type of brain malignancy in adults. It is considered the most aggressive and lethal form of brain tumor, with overall survival of approximately 15 months after diagnosis. Gliomas are the most common type of central nervous system (CNS) malignancy and start in the glial cells (non-neuronal CNS cells) from the brain or spine. Glioma cell lines serve as useful tools for studying the cell biology of brain tumors. The SNB-75 cell line was isolated from a Grade IV glioblastoma obtained in 1980 from a 78-year-old female patient. It is known for being a part of the NCI-60 screening program. In a 1988 study, published in Cancer Research Journal, the SNB-75 cell line was moderately clonogenic in soft agar, showed tumorigenicity in nude mice and secreted plasminogen activator. A 1994 Cancer Research article (Plowman et al.) used SNB-75 cells to study the antitumor activity of temozolomide. The group monitored treatment on SNB-75 xenografted mice and found that there was a synergistic effect between temozolomide and 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU). A 2017 Cancer Research study (Holbeck et al.) used 67 cancer lines, including SNB-75 cells, to evaluate over 5,000 pairings of FDA-approved anticancer drugs. The results were compiled into the NCI-ALMANAC database and was overall useful in identifying clinically relevant anticancer drug combinations. Human tumor xenografting is essential in finding novel treatment therapies for improving the survival rate of brain cancer patients. The SNB-75 cell line is used to create the cell line derived SNB-75 xenograft mouse model. The SNB-75 xenograft model is a functional animal model used to develop novel monotherapies along with combinatorial studies to determine pre-clinical antitumor synergism with temozolomide (e.g. BCNU).
Download Altogen Labs SNB75 Xenograft Model PowerPoint Presentation: ![]()
Basic study design
- 10 to 12 w.o. athymic BALB/C or NOD/SCID receive single injections into the flank of the hind leg. Each injection contains one million cells of the 50% Matrigel plus SNB-75 cell suspension (in a volume of 0.1 ml).
- The injection sites are watched and palpated until tumors are deemed established. Tumors are assessed (digital calipers) until they reach average sizes in the range of 120-150 mm3. Treatment groups are established by randomization. The compound of interest is injected according to customer supplied dosing schedule.
- In-life tumor measurements are taken daily, with whole body weights tracked 3 times weekly.
- Maximum permitted tumor size determines the end of the study, at which time necropsies and tissue collections are performed.
- Tumor samples are excised and weighed. Tumor samples can also be documented by digital imaging. All tissues collected are stored according to customer indication (snap frozen in liquid nitrogen, immersed in 10% NBF formalin solution, etc).
Get Instant Quote for
SNB-75 Xenograft Model
SNB-75 cells have been extensively characterized and are known to harbor several genetic mutations, including mutations in the tumor suppressor gene p53 and the oncogene EGFR. These mutations are commonly found in glioblastoma tumors and are thought to contribute to tumor growth and resistance to therapy. SNB-75 cells are also known to express high levels of the enzyme carbonic anhydrase 12, which has been implicated in the growth and invasiveness of glioblastoma tumors. Xenograft animal models are used to assess the effectiveness of test compounds against specific types of cancer. New medicines are tested on staged tumor growths that have been engrafted via subcutaneous or orthotopic inoculation in an immunocompromised mouse or rat model. Animal handling and maintenance at the Altogen Labs facilities are IACUC regulated and GLP compliant. Following acclimation to the vivarium environment, mice are sorted according to body mass. The animals are examined daily for tumor appearance and clinical signs. We provide detailed experimental procedures, health reports and data (all-inclusive report is provided to the client that includes methods, results, discussion and raw data along with statistical analysis). Additional services available include collection of tissue, histology, isolation of total protein or RNA and analysis of gene expression. Our animal facilities have the flexibility to use specialized food or water systems for inducible gene expression systems.
Following options are available for the SNB-75 xenograft model:
- SNB-75 Tumor Growth Delay (TGD; latency)
- SNB-75 Tumor Growth Inhibition (TGI)
- Dosing frequency and duration of dose administration
- Dosing route
- SNB-75 tumor immunohistochemistry
- Alternative cell engraftment sites (orthotopic transplantation)
- Blood chemistry analysis
- Toxicity and survival (optional: performing a broad health observation program)
- Gross necropsies and tissue pathology analysis
- Positive control group employing cyclophosphamide, at a dosage of 50 mg/kg administered by intramuscular injection to the control group daily for the study duration
- Lipid distribution and metabolic assays
- Imaging studies: Fluorescence-based whole body imaging, MRI
