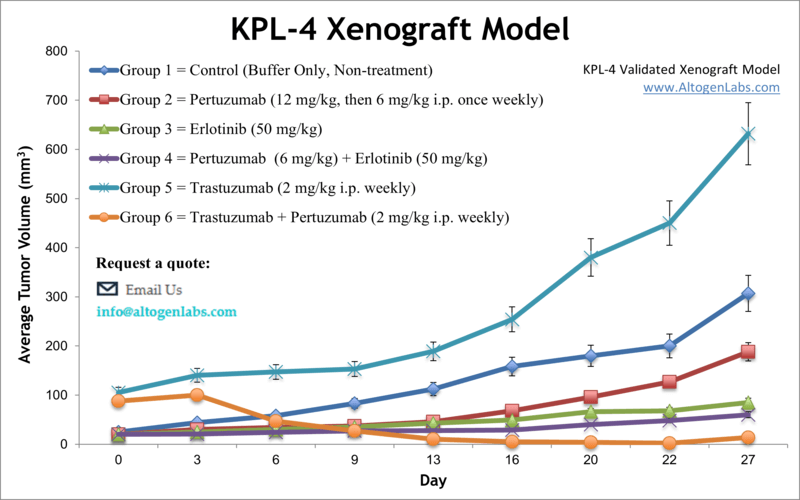
KPL-4 xenograft model
KPL-4 is a human breast cancer cell line that was originally derived from a breast cancer patient with metastasis to the axillary lymph node. These cells have been widely used as a model system for studying various aspects of breast cancer biology, including the molecular mechanisms underlying tumor development and progression, and the identification of potential therapeutic targets for the treatment of breast cancer. KPL4 cells have several properties that make them useful for research. Firstly, they are relatively easy to culture in the laboratory and can be grown in large quantities. Secondly, they express markers of breast cancer, such as estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2), and exhibit several features characteristic of breast cancer, including uncontrolled proliferation, invasion, and metastasis. Thirdly, they respond to various stimuli, such as hormones and growth factors, making them a useful model system for studying the effects of these factors on breast cancer cell growth and survival.
Breast cancer represents an increasing public health problem as this is the most common cancer in females worldwide, with nearly 1.7 million of new cases detected annually. The KPL-4 cell line was established using the malignant pleural effusion of a breast cancer patient with skin metastasis and has been extensively employed in breast cancer research. According to a 1999 study, published in British Journal of Cancer, the KPL-4 cell line is tumorigenic and readily metastasizes into the lymph nodes and lungs. KPL-4 cells express Erb-B family receptors and have proven to be invaluable models for studying the cell biology of aggressive breast cancers. The KPL-4 model of breast cancer has been used in many studies including one by Suzuki et al. (2009) where the group investigated novel therapies to overcome mutation-mediated resistance to inhibitors of epidermal growth factor receptor (EGFR) kinase, which are a common clinical therapy regimen. Results showed potential for the dual kinase inhibitor MP-412 (aka AV-412), which targets EGFR and ErbB2, and found that it inhibited tumor growth in vivo in otherwise resistant solid tumor models. A 2009 study by Sheuer et al. used KPL-4 to examine the combination treatment of trastuzumab and pertuzumab against human epidermal growth factor receptor 2 (HER2) positive metastatic breast cancers; their results suggested that this combination was a success in inhibiting tumor growth and inducing tumor regression in vivo because of the differing mechanisms of action between the compounds of inhibition of HER2 dimerization (trastuzumab) and prevention of p95HER2 formation (pertuzumab). Finally, Pastuskovas et al. released a study in 2012 investigating the pharmacokinetics of combination treatment of trastuzumab (HER2 inhibitor) and bevacizumab (VEGF inhibitor); they looked into autoradiography, biodistribution and SPECT-CT imaging in a KPL-4 xenograft tumor model and showed that bevacizumab prevented uptake of trastuzumab into tumors which would prevent a favorable clinical outcome, serving as a caution when designing combination therapies and dosing strategies. The KPL4 cell line is used to create the xenograft mouse model. Examination of antitumor activity of monotherapies or combination studies in the KPL4 xenograft model include erlotinib, capecitabine or anti-ErbB2 agents.
Download Altogen Labs KPL-4 Xenograft Model PowerPoint Presentation: ![]()
Basic study design
- Exponential growth of cells are under aseptic conditions prior to collection.
- All cells are collected for inoculation and viability determined. Trypan blue exclusion is utilized with a 98% minimum viability required.
- Cell suspensions are adjusted such that 100 µL of the Matrigel+KPL-4 cell suspension contains one million cells. Cells are inoculated s.c. into the flank of a hind leg per mouse. The mice are NOD/SCID or athymic BALB/C and are 10 to 12 weeks old.
- Calipers are used for tumor monitoring, with expectation of 50-150 mm3 tumors needed to start the study. Animals are sorted into cohorts and compounds are administered according to treatment schedule.
- Daily tumor measurements mouse weights are recorded until the tumor size limit is reached. A necropsy is performed to remove tumors, record their weight and then document digitally.
- Tissues are submersed in RNA-later, snap frozen, nucleic acids isolated or tissues prepared for histological analysis.
Get Instant Quote for
KPL-4 Xenograft Model
Xenograft animal models are used to assess the effectiveness of drugs against specific types of cancer. New medicines are tested on staged tumor growths that have been engrafted via subcutaneous or orthotopic inoculation in an immunocompromised mouse or rat model. All clinically approved anti-cancer agents have been evaluated with conventional preclinical in vivo models. Xenograft studies can be highly complex, starting with the selection of the appropriate animal model, choice of tumorigenic cell line, administration method, dosing, analysis of tumor growth rates and tumor analysis (histology, mRNA and protein expression levels).
Altogen Labs provides an array of laboratory services using over 90 standard Cell Line Derived Xenograft (CDX) models and over 30 PDX models. Researchers investigating the role of specific proteins or gene products in regulating tumor growth can benefit from development of protein overexpression (genetically engineered to ectopically express proteins, tumor suppressors, or oncogenes) and RNAi cell lines with long term gene silencing. Altogen Labs provides quantitative gene expression analysis of mRNA expression (RT-PCR) and protein expression analysis using the WES system (ProteinSimple).
Animal handling and maintenance at the Altogen Labs facility is IACUC-regulated and GLP-compliant. Following acclimatization to the vivarium environment, mice are sorted according to body mass. The animals are examined daily for tumor appearance and clinical signs. We provide detailed experimental procedures, health reports and data (all-inclusive report is provided to the client that includes methods, results, discussion and raw data along with statistical analysis). Additional services available include collection of tissue, histology, isolation of total protein or RNA and analysis of gene expression. Our animal facilities have the flexibility to use specialized food or water systems for inducible gene expression systems.
Following options are available for the KPL-4 xenograft model:
- KPL-4 Tumor Growth Delay (TGD; latency)
- KPL-4 Tumor Growth Inhibition (TGI)
- Dosing frequency and duration of dose administration
- Dosing route (intravenous, intratracheal, continuous infusion, intraperitoneal, intratumoral, oral gavage, topical, intramuscular, subcutaneous, intranasal, using cutting-edge micro-injection techniques and pump-controlled IV injection)
- KPL-4 tumor immunohistochemistry
- Alternative cell engraftment sites (orthotopic transplantation, tail vein injection and left ventricular injection for metastasis studies, injection into the mammary fat pad, intraperitoneal injection)
- Blood chemistry analysis
- Toxicity and survival (optional: performing a broad health observation program)
- Gross necropsies and histopathology
- Positive control group employing cyclophosphamide, at a dosage of 50 mg/kg administered by intramuscular injection to the control group daily for the study duration
- Lipid distribution and metabolic assays
- Imaging studies: Fluorescence-based whole body imaging
