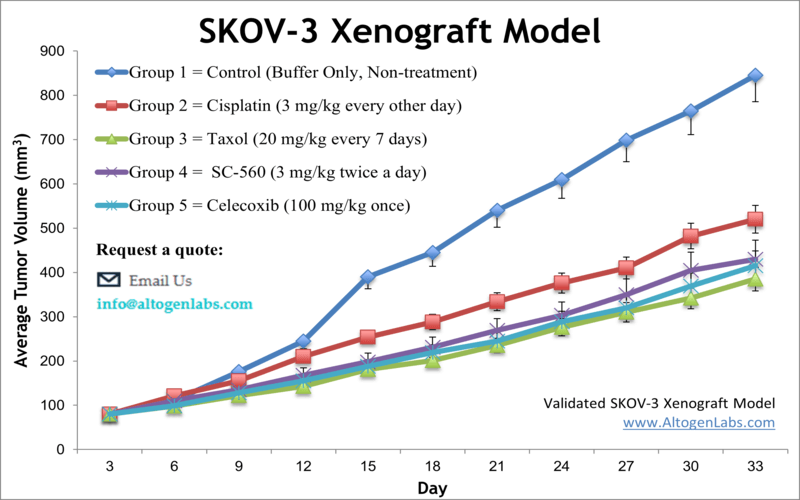
SKOV3 xenograft model
SKOV3 cells are known to exhibit many of the characteristics of ovarian cancer, including abnormal cell growth and differentiation, resistance to apoptosis (programmed cell death), and the ability to invade surrounding tissue. Ovarian cancer is the deadliest gynecological malignancy that is often asymptomatic at early stages. The vast majority of the patients are already at the advanced stages of the disease at the time of diagnosis. The SK-OV-3 tumorigenic epithelial cell line was isolated in 1973 from the ovarian tissue of a 64-year-old Caucasian female patient with adenocarcinoma. SK-OV-3 is resistant to tumor necrosis and several other cytotoxic drugs, including Adriamycin, cis-platinum and diphtheria toxin. SK-OV-3 is hypodiploid and a suitable transfection host for ovarian cancer research. Integrin-associated protein (IAP) CD47 is a membrane protein of the immunoglobulin superfamily that is a proven ovarian cancer marker. CD47 is overexpressed in ovarian cancer cells and indicates a poor prognosis. A 2017 study by Liu et al. published in Oncotarget, reported that CD47 knockdown in the SK-OV-3 ovarian cancer cell line promoted phagocytosis by macrophages in vitro and blocked tumor growth in vivo in the SK-OV-3 xenograft model. These findings indicate that CD47 inhibition could be a potential strategy for the treatment of ovarian cancer patients. Li et al. published a study in the Journal of Cancer Research and Therapeutics using the SKOV3 xenograft model to study bevacizumab resistance mechanisms in ovarian cancer. Results demonstrated an overexpression of EphB4 in bevacizumab resistant tumors; combination treatment with NVP-BHG712, an EphB4 inhibitor, enhanced antitumor activity of bevacizumab. These findings have clinical relevance as a potential treatment for bevacizumab resistant patients. In 2008, Molecular Cancer Therapeutics published an article by Vassileva et al. which investigated the effects of sustained as compared to intermittent paclitaxel treatment in an SKOV-3 model of ovarian cancer. The study looked at tumor proliferation, growth and apoptosis in immunoassays; results demonstrated that intermittent treatment, which is a common occurrence in clinical treatment strategies, led to increased proliferation, repopulation and clonogenic survival whereas sustained paclitaxel treatment did not decrease survival but did have a higher ratio of apoptotic cells. For clinical translation, this may mean that sustained chemotherapy may attenuate tumor repopulation and increase chemoresponsiveness. Finally, Yu et al. (2014) used the SKOV3 xenograft model to study the antitumor effects of an antibody-cytotoxic drug conjugate of trastuzumab (trastuzumab-DM1, T-DM1) on HER2-positive ovarian cancer. Results demonstrated T-DM1 led to significant tumor growth inhibition, which supports further investigation of T-DM1 as an anticancer therapeutic strategy. The SK-OV-3 cell line (human ovarian) is used to create the CDX (Cell Line Derived Xenograft) SKOV-3 xenograft mouse model. The SK-OV-3 xenograft model exhibits the following features:
- Clinical efficacy of taxol is well documented and allows for combination therapies of novel compounds
- High HER2-positive expression leads to antitumor activity of HER2 targeted therapies (e.g. trastuzumab-DM1, T-DM1)
- Utilized for the study of epothilone analogues, which are natural product-based antitumor drugs (e.g. iso-dehydelone)
Download Altogen Labs SK-OV-3 Xenograft Model PowerPoint Presentation: ![]()
Basic study design
- Athymic nu/nu mice (10 to 12 weeks) are inoculated with a subcutaneous injection of 1 x 106 cells plus Matrigel in the hind leg (injection volume = 100 µL).
- Tumors are calipered (digital calipers) and grouped into treatment cohorts with an average size of 50-150 mm3. Administration of test compounds is performed according to client supplied treatment schedule.
- Whole body mouse weights are logged up to 3 times a week. Tumor sizes are measured per client instructions (typically daily).
- The in-life portion of the study is completed as tumor size reaches the client supplied tumor size limit (or a maximum of 2,000 mm3). Tissue collection proceeds following the termination of experiment.
- All remaining tumors are excised from the mice and weighed. Standard gross necropsies allow for the collection of tissue requested by the client (options include snap frozen, histology preparation in 10% NBF or stabilized in RNAlater reagent).
Get Instant Quote for
SK-OV-3 Xenograft Model
Xenograft animal models are used to assess the effectiveness of drugs against specific types of cancer. New medicines are tested on staged tumor growths that have been engrafted via subcutaneous or orthotopic inoculation in an immunocompromised mouse or rat model. All clinically approved anti-cancer agents have been evaluated with conventional preclinical in vivo models. Xenograft studies can be highly complex, starting with the selection of the appropriate animal model, choice of tumorigenic cell line, administration method, dosing, analysis of tumor growth rates and tumor analysis (histology, mRNA and protein expression levels).
Altogen Labs provides an array of laboratory services using over 90 validated CDX and 30 PDX models. Researchers investigating the role of specific proteins or gene products in regulating tumor growth can benefit from development of protein overexpression (genetically engineered to ectopically express proteins, tumor suppressors, or oncogenes) and RNAi cell lines with long term gene silencing. Altogen Labs provides quantitative gene expression analysis of mRNA expression (RT-PCR) and protein expression analysis using the WES system (ProteinSimple).
Animal handling and maintenance at the Altogen Labs facility is IACUC regulated and GLP compliant. Following acclimation to the vivarium environment, mice are sorted according to body mass. The animals are examined daily for tumor appearance and clinical signs. We provide detailed experimental procedures, health reports and data (all-inclusive report is provided to the client that includes methods, results, discussion and raw data along with statistical analysis). Additional services available include collection of tissue, histology, isolation of total protein or RNA and analysis of gene expression. Our animal facilities have the flexibility to use specialized food or water systems for inducible gene expression systems.
Following options are available for the SK-OV-3 xenograft model:
- SK-OV-3 Tumor Growth Delay (TGD; latency)
- SK-OV-3 Tumor Growth Inhibition (TGI)
- Dosing frequency and duration of dose administration
- Dosing route (intravenous, intratracheal, continuous infusion, intraperitoneal, intratumoral, oral gavage, topical, intramuscular, subcutaneous, intranasal, using cutting-edge micro-injection techniques and pump-controlled IV injection)
- SK-OV-3 tumor immunohistochemistry
- Alternative cell engraftment sites (orthotopic transplantation, tail vein injection and left ventricular injection for metastasis studies, injection into the mammary fat pad, intraperitoneal injection)
- Blood chemistry analysis
- Toxicity and survival (optional: performing a broad health observation program)
- Gross necropsies and histopathology
- Positive control group employing cyclophosphamide, at a dosage of 20-30 mg/kg
