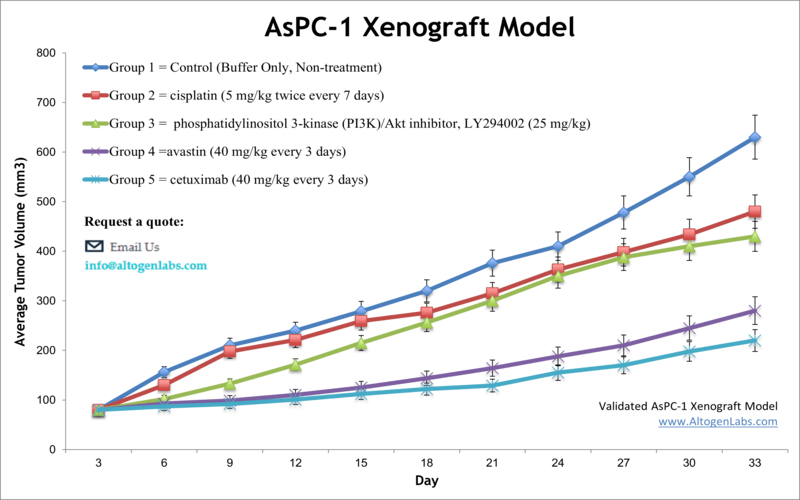
AsPC1 xenograft model
ASPC-1 is a human pancreatic cancer cell line that was originally isolated from a patient with pancreatic adenocarcinoma in 1982. Pancreatic cancer is a fatal disease and the third leading cause of cancer death in the United States. Despite recent advances in chemotherapeutic approaches, a 5-year survival for pancreatic cancer is less than 7 percent for all stages, which makes it a significant public health challenge. The AsPC-1 epithelial cell line is isolated from pancreatic tissue of a 62-year-old Caucasian female patient with metastatic pancreatic adenocarcinoma. AsPC1 is extensively employed for studying human infections related to the pancreas. A 2016 study by Henderson published in Neoplasia investigated the efficacy of a novel histone deacetylase (HDAC) inhibitor AR-42 in suppressing tumor growth in the AsPC-1 model. Results showed tumor suppression associated with HDAC inhibition and increased apoptosis is observed in AR-42-treated cells both in vivo and in vitro. These findings indicate that AR-42 could be a promising therapeutic approach for the treatment of pancreatic cancer. A 2016 Nature article (Huang et al.) used the AsPC-1 cell line and xenograft to investigate the mechanism of action of LTP-1, an antimitotic and STAT3 inhibitor. Results demonstrated that treatment of LTP-1 led to cell cycle arrest, microtubule dynamic disruption, Stat3 dephosphorylation, ERK activation, caspase activation, and tumor growth suppression. This supports LTP-1 for further study as a clinical therapeutic agent. Another study that used AsPC-1 cells is the 2006 International Journal of Cancer article (Ito et al.) which characterized the mTOR inhibitor CCI-779 in human pancreatic cancer. Results demonstrated CCI-779 led to Akt, mTOR and S6K1 activation as well as antitumor effects in vivo. Today, CCI-779 is known as temsirolimus (Torisel), is FDA approved, and is used to treat advanced kidney cancer. In 2014 Inagawa et al. (Carcinogenesis) used various pancreatic cell lines, including AsPC-1, to establish carcinogenesis models of pancreatic ductal adenocarcinomas (PDACs). They successfully transformed normal human pancreatic duct epithelial cells (HPDECs) into adenocarcinomas by manipulating key oncogenes including KRAS, p53 and p16-pRB, which may have implications to carcinogenesis across various organs. The AsPC-1 cell line (human pancreas) is used to create the CDX (Cell Line Derived Xenograft) AsPC-1 xenograft mouse model. This model enables the in vivo study of cisplatin, avastin or cetuximab in an AsPC-1 CDX model.
Download Altogen Labs AsPC1 Xenograft Model PowerPoint Presentation: ![]()
Basic study design
- AsPC-1 cells used for injection are maintained under exponential phase growth.
- Prior to injection, the cells are prepared by trypsinization and viable cell counts determined using trypan blue exclusion (required 98-99% cell viability). The cell suspension is adjusted to the appropriate density.
- Each mouse (NOD/SCID or athymic BALB/C, 10-12 weeks old) receives a single subcutaneous injection in the hind leg flank containing 1 million cells (100-150 µL of the AsPC-1 cell suspension in Matrigel).
- Injection sites are palpated multiple times weekly until establishment of tumors. Tumors are measured with digital calipers to obtain an average size of 50-150 mm3.
- Randomization into treatment cohorts and administration of the test compound is performed according to the agreed upon treatment schedule.
- Tumors are measured daily with mouse weights being recorded 2-3 times weekly.
- Animals are euthanized as tumor size reaches 2,000 mm3 or the predetermined size limit by IACUC protocol. The necropsy is performed as defined in the termination of the experiment.
- The tumors are excised, weighed and then documented via digital imaging.
- Standard gross necropsies and tissue collection is performed for downstream analysis.
- Tumors and tissues can be stabilized in RNA-later, snap frozen in LN2, prepared for histology or go through an RNA isolation for gene expression analysis.
Get Instant Quote for
AsPC-1 Xenograft Model
Xenograft animal models are used to assess the effectiveness of drugs against specific types of cancer. New medicines are tested on staged tumor growths that have been engrafted via subcutaneous or orthotopic inoculation in an immunocompromised mouse or rat model. All clinically approved anti-cancer agents have been evaluated with conventional preclinical in vivo models. Xenograft studies can be highly complex, starting with the selection of the appropriate animal model, choice of tumorigenic cell line, administration method, dosing, analysis of tumor growth rates and tumor analysis (histology, mRNA and protein expression levels). The dosing of the experimental compound of interest is initiated, for a staged study, when the mean tumor size reaches a specified volume (typically 50-100 mm3). Animal handling and maintenance at the Altogen Labs facility is IACUC regulated and GLP compliant. Following acclimatization to the vivarium environment, mice are sorted according to body mass. The animals are examined daily for tumor appearance and clinical signs. We provide detailed experimental procedures, health reports and data (all-inclusive report is provided to the client that includes methods, results, discussion and raw data along with statistical analysis).
Following options are available for the AsPC-1 xenograft model:
- AsPC-1 Tumor Growth Delay (TGD; latency)
- AsPC-1 Tumor Growth Inhibition (TGI)
- Dosing frequency and duration of dose administration
- Dosing route (intravenous, intratracheal, continuous infusion, intraperitoneal, intratumoral, oral gavage, topical, intramuscular, subcutaneous, intranasal, using cutting-edge micro-injection techniques and pump-controlled IV injection)
- AsPC1 tumor pathology and immunohistochemistry
- Alternative cell engraftment sites (orthotopic transplantation, tail vein injection and left ventricular injection for metastasis studies, injection into the mammary fat pad, intraperitoneal injection)
- Blood chemistry analysis
- Toxicity and survival (optional: performing a broad health observation program)
- Gross necropsies and histopathology
- Positive control group employing known chemotherapy treatment
- Imaging studies: Fluorescence-based whole body imaging
