U-87 MG xenograft model
The U87MG xenograft model is one of the most widely utilized systems for studying glioblastoma multiforme (GBM), a highly aggressive and fatal primary brain tumor in adults. Originally derived in 1966 at Uppsala University from a 44-year-old female patient, the U87MG cell line has become a foundational tool in neuro-oncology research. Although later genetic analyses revealed that the currently distributed U87MG cells differ from the original tumor tissue, they retain key glioblastoma characteristics and remain a reliable model for preclinical studies. U87MG cells are characterized by several defining genetic features. They carry a homozygous deletion in the PTEN tumor suppressor gene, resulting in constitutive activation of the PI3K/Akt pathway. The TP53 gene remains wild-type, enabling p53-mediated apoptotic responses. The cell line also lacks expression of MGMT due to promoter methylation, rendering it particularly sensitive to alkylating agents such as temozolomide. U87MG exhibits numerous chromosomal abnormalities and a hyperdiploid karyotype, with deletions in loci such as CDKN2A that are consistent with common alterations in human GBM. In vivo, U87MG xenografts are generated by implanting cells into immunocompromised mice. Subcutaneous implantation in the flank allows for straightforward tumor measurement and drug testing, while orthotopic implantation into the brain provides a more clinically relevant environment. Subcutaneous tumors typically grow as vascularized, encapsulated masses with pseudopalisading necrosis, while orthotopic tumors remain well-circumscribed and non-invasive, differing from the infiltrative growth of patient GBMs. Both models demonstrate strong angiogenic activity, partly due to PTEN loss and high VEGF production. Despite limitations such as a lack of true invasion and clonal homogeneity, U87MG xenografts have proven highly valuable in assessing therapeutic efficacy. They have been widely used to test temozolomide, VEGF inhibitors, PI3K/Akt pathway inhibitors, and combination therapies involving MDM2 antagonists. Engineered variants of U87MG have also facilitated studies on EGFR-targeted therapies and CAR T cell approaches. The model’s predictability and consistent tumor formation have supported its use in drug development and mechanistic studies. In addition to therapeutic evaluations, U87MG xenografts are used for preclinical imaging research. Tumors formed by these cells have been instrumental in developing and validating MRI, PET, and optical imaging modalities that assess tumor vascularity, metabolic activity, and response to treatment. Bioluminescent U87-MG models are especially useful for non-invasive monitoring of tumor burden in live animals. While the U87MG model does not fully recapitulate the complex biology of GBM, it remains a cornerstone of glioblastoma research. Its genetic profile, reproducible tumorigenicity, and long history of experimental use have established its relevance, particularly in studies of angiogenesis, tumor metabolism, and therapeutic response. Used with awareness of its limitations, the U87 xenograft model continues to contribute meaningful insights into GBM pathophysiology and treatment strategies.
U87 Brain Cancer Xenograft Model: Download ![]()
Download Altogen Labs U87 Xenograft Model PowerPoint Presentation: ![]()
U87 Subcutaneous Xenografts in Research
Subcutaneous xenograft transplantation using the U87 glioblastoma cell line is a widely employed preclinical model for studying tumor growth, therapeutic response, and resistance mechanisms in glioma research. Derived from a human astrocytoma, U87 cells readily form vascularized, well-demarcated tumors when injected subcutaneously into immunodeficient mice, enabling reproducible and accessible in vivo experimentation. This model has been instrumental in evaluating chemotherapeutics such as temozolomide and targeted agents including VEGF and EGFR inhibitors, particularly in the context of angiogenesis. Despite its experimental utility, the model lacks key features of clinical glioblastoma, including invasive growth and microenvironmental complexity, and concerns have been raised regarding genetic drift and divergence from the original tumor phenotype. These limitations necessitate cautious interpretation of results and often warrant complementary use of orthotopic or patient-derived models. Altogen Labs offers an in-house validated U87 subcutaneous xenograft model, supporting early-phase oncology studies with standardized protocols and comprehensive analytical endpoints.
Intracranial Glioblastoma Modeling Using U87 Cells (Orthotopic U87 Model)
Orthotopic xenograft transplantation using the U87 glioblastoma cell line offers a biologically relevant model for studying tumor progression, therapeutic response, and intracranial drug delivery within the native brain environment. Stereotactic injection of U87 cells into the striatum or cortex of immunodeficient mice enables controlled tumor formation that reflects key aspects of glioblastoma localization, facilitating longitudinal analysis using imaging and histopathology. While U87-derived tumors in this setting lack the diffuse infiltration characteristic of primary glioblastomas, they are well-suited for assessing drug penetration across the blood-brain barrier and the efficacy of targeted treatments. Notable studies have shown that genetic manipulations, such as SNORD47 overexpression and HOTAIR knockdown, can significantly alter tumor behavior and survival outcomes in orthotopic models, while additional research has demonstrated the feasibility of advanced delivery methods like focused ultrasound-enhanced chemotherapy. Although the model has limitations related to invasiveness and tumor heterogeneity, it remains widely used in preclinical glioblastoma research due to its reproducibility and relevance for evaluating intracranial therapeutic strategies.
U87 Glioma Cells Drive Tau Aggregation via Soluble CD44 Secretion
In a study published in Experimental & Molecular Medicine journal, Lim et al. investigated a mechanistic link between glioblastoma pathology and neurodegeneration, using the U87 cell line to establish a xenograft model and secretome system. The central finding of the study is that soluble CD44 (sCD44), secreted by U87 cells, induces tau hyperphosphorylation and aggregation, which in turn promotes neuronal degeneration. U87-conditioned media significantly increased tau aggregation in tau-BiFC reporter cells, elevated phospho-tau levels at Ser199 and Ser396, and shortened neurite outgrowth in primary rat hippocampal neurons. Secretome profiling through FPLC and mass spectrometry identified sCD44, SPARC, and LDHA as candidate proteins, but only siRNA-mediated knockdown of CD44 in U87 cells abolished tau-inducing activity. Direct injection of purified sCD44 into the hippocampus of tau transgenic mice further validated its neurodegenerative effect, as shown by increased AT8-positive neurons and elevated tau pathology in brain regions distal to the tumor. Notably, U87-derived sCD44 was found to be spatially correlated with sites of tau aggregation in the xenografted brains. The data reveal a consistent pattern in which U87 cells, through high CD44 expression and secretion of sCD44, drive tau pathology both in vitro and in vivo. The correlation between regions of sCD44 accumulation and tau aggregation strongly supports the paper’s hypothesis that glioblastoma-secreted factors can propagate neurodegenerative changes. Methodologically, the study benefits from the use of multiple glioblastoma lines (including U87, T98G, HS683), appropriate controls, and both in vitro and in vivo models, thereby enhancing the robustness of the findings. However, the reliance on a single glioblastoma xenograft model (U87) limits generalizability, given U87’s well-documented lack of infiltrative behavior and limited resemblance to primary GBM heterogeneity. Furthermore, while the use of tau-BiFC sensor cells provides a sensitive readout for aggregation, it may not fully capture the complexity of tau pathology in vivo. Nonetheless, the implications are substantial: the identification of sCD44 as a glioblastoma-derived mediator of tauopathy opens new avenues for understanding the intersection between brain tumors and neurodegeneration. Future studies should assess whether sCD44 plays similar roles in patient-derived xenografts or infiltrative glioma models and whether blocking CD44 cleavage can mitigate glioma-induced neuronal damage.
Overcoming TMZ Resistance in U87 Xenografts with SN-38 Delivery
Temozolomide (TMZ) is a first-line chemotherapeutic agent for glioblastoma, but its effectiveness is often limited by acquired resistance. In contrast, SN-38, the active metabolite of irinotecan, exerts antitumor effects through topoisomerase I inhibition, offering a mechanistically distinct approach. In glioblastoma models utilizing U87 and TMZ-resistant U87-TR cells, combining SN-38 with TMZ significantly improves therapeutic outcomes. When SN-38 is delivered via biodegradable polymeric microparticles for sustained intracranial release, it induces potent cytotoxic effects in U87 cells with nanomolar-range IC50 values that decrease over time. In both TMZ-sensitive and TMZ-resistant U87-derived intracranial xenografts, combination therapy markedly reduces tumor growth, suppresses cellular proliferation, and enhances apoptosis. Bioluminescence imaging and survival analysis consistently show superior outcomes compared to standard treatments. Histopathological assessments reveal reduced central necrosis and increased GFAP expression, indicating diminished malignancy and possible re-differentiation of glioma cells. Notably, this regimen also prevents spinal metastases in aggressive U87-TR models, suggesting improved control of tumor dissemination. These results support the rationale for combining mechanistically distinct agents using localized delivery systems to overcome resistance and enhance efficacy in glioblastoma, with U87 models serving as a valuable platform for preclinical evaluation.
RND1 Suppresses U87 Tumor Progression via AKT Signaling
The U87 glioblastoma cell line, widely used in preclinical studies, plays a central role in elucidating molecular mechanisms underlying tumor progression and chemoresistance. In this context, the RND1 gene has emerged as a key regulator of epithelial-mesenchymal transition (EMT) and temozolomide (TMZ) sensitivity. Overexpression of RND1 in U87 cells led to significant reductions in cell proliferation, migration, and colony formation. These phenotypic changes were accompanied by increased expression of anti-EMT markers such as E-cadherin and decreased levels of pro-EMT proteins including N-cadherin, vimentin, Snail1, and MMP2. Knockdown of RND1 reversed these effects, enhancing tumor-like behaviors and promoting EMT. At the signaling level, RND1 suppressed phosphorylation of AKT and its downstream effector GSK3β, identifying the AKT/GSK3β axis as a critical mediator of its tumor-suppressive function. Notably, U87 cells rendered resistant to TMZ (U87-TR) exhibited heightened AKT signaling and mesenchymal features, both of which were mitigated by RND1 overexpression. Pharmacological activation of AKT abrogated these effects, confirming the pathway’s involvement. In vivo, RND1 overexpression suppressed tumor growth and improved TMZ responsiveness in xenograft models. These findings support a model in which RND1 inhibits oncogenic behavior in U87 cells by modulating EMT through AKT signaling and sensitizing cells to TMZ, underscoring its relevance as a potential therapeutic target in glioblastoma.
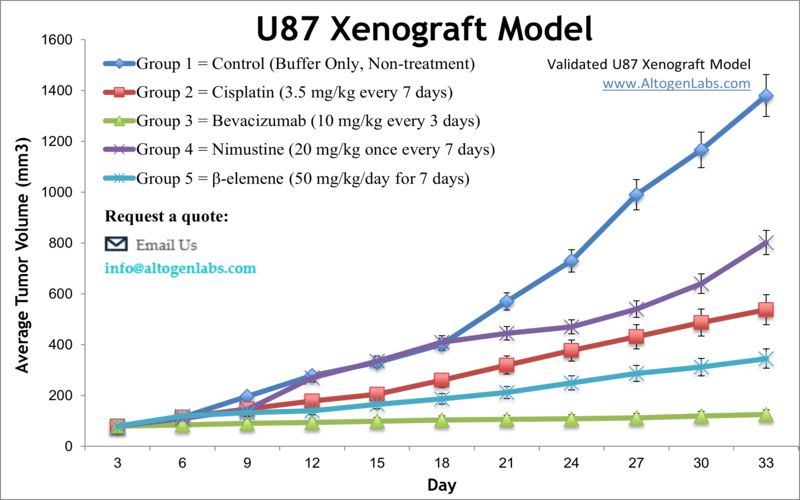
Basic study design
- Exponential growth of cells is maintained for U87 cells prior to injection. Trypsin-EDTA is used to collect cells, which is followed by trypan blue (cell viability) cell counting. The cell concentration is then diluted to 10,000 cells/µL.
- Twelve (12) weeks old athymic BALB/c nude mice receive an s.c. injection. All inoculations are made into the hind leg and contain Matrigel plus U-87 MG cells. Tumor measurements are made using calipers. Upon averages of 120-150 mm3, the animals are grouped into the number of treatment cohorts needed for the study. In-life dosing of all test compounds are performed following the client supplied schedule.
- Continual tumor measurements (e.g. daily) and whole body weights are logged. At the end of the study, tumors are weighed and then documented (optional digital imaging).
- In preparation for future downstream analysis, tumors/tissues are stored according to client instructions, including options such as samples being stabilized (RNAlater), snap frozen or prepared for histology (10% NBF).
Get Instant Quote for
U-87 MG Xenograft Model
U87-MG is a human glioblastoma cell line that was derived from a malignant brain tumor in a patient. The cells are widely used in research as a model for studying the biology of glioblastoma and testing potential treatments for the disease. U87MG cells have been extensively characterized and are known to harbor several genetic mutations, including mutations in the tumor suppressor gene p53 and the oncogene EGFR. These mutations are commonly found in glioblastoma tumors and are thought to contribute to tumor growth and resistance to therapy. One of the notable features of U87MG cells is their ability to invade surrounding tissues, a property that is typical of glioblastoma tumors in patients. U87MG cells are also used as a model system for testing potential glioblastoma therapies.
U87 Brain Cancer Xenograft Model: Download ![]()
Following options are available for the U87 xenograft model:
- U87 Tumor Growth Delay (TGD; latency)
- U87 Tumor Growth Inhibition (TGI)
- Dosing frequency and duration of dose administration
- U87 tumor immunohistochemistry
- Alternative cell engraftment sites (orthotopic transplantation)
- Blood chemistry analysis
- Toxicity and survival (optional: performing a broad health observation program)
- Gross necropsies and histopathology
- Imaging studies: Fluorescence-based whole body imaging
