The LN-229 human brain glioblastoma cell line was first obtained in 1979 from a patient with right frontal parieto-occipital glioblastoma. The LN-229 cell line has a wild-type PTEN gene, mutated p53, and may potentially have homozygous deletions in the p16 as well as the p14ARF tumor suppressor genes. In a 1997 study, published in BBA Molecular Cell Research Journal, the LN-229 cell line was evaluated after the treatment with puromycin that killed LN-229 cells in a dose-dependent manner. In addition, stimulation of these cells with Fas ligand lead to apoptotic cell death within 16 hours. A 2011 Cancer Research study identified the LN-229 rodent xenograft model as a valid system to use the cryo-imaging technique for examining tumor progression and metastasis. This method differs from the traditional methods of serial sections and histological stains of brain tissue in that it uses computer algorithms to reconstruct 3-D images of this glioma model, essentially improving resolution and allowing the study of tumor cell invasion and dispersal. The LN-229 xenograft model has also been used in a 2013 study by Grommes et al. to identify the proliferator-activated receptor gamma (PRARγ) small molecule agonist pioglitazone as a potential therapeutic treatment for malignant gliomas. Pioglitazone was shown to cross the blood-brain barrier and exhibit antineoplastic effects in the LN-229 glioblastoma xenograft model. The 2012 study by Chen et al also used the LN-229 cell line; this group identified a brain specific micro-RNA, miR-524-5p, that acts as a tumor suppressor. They demonstrated the targeting of Jagged-1 and Hes-1, key components of stem cell maintenance and angiogenesis pathways, by miR-524-5p and subsequent suppression of cell proliferation and invasion upon the miRNA’s restoration.
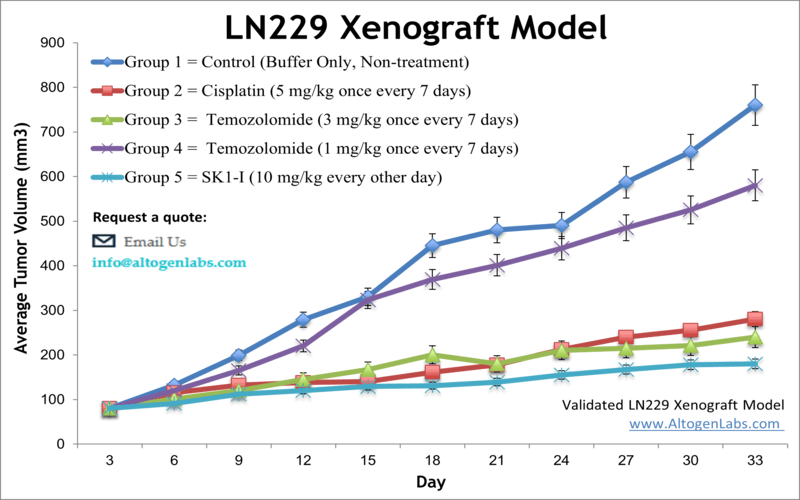
Altogen Labs provides xenotransplantation services for LN-229 glioblastoma cells to study tumor formation and progression. The LN-229 cell line is used to create the CDX (Cell Line Derived Xenograft) LN229 xenograft mouse model. Targeting S1P to induce apoptosis (e.g. SK1-I) and inhibit AKT signaling are some of the uses of the LN-229 xenograft model, including tumor growth suppression with chemotherapies (e.g. temozolomide or cisplatin).
Download Altogen Labs LN-229 Xenograft Model PowerPoint Presentation: ![]()
Basic study design
- Exponentially growing cells are collected for inoculation with viability determined by trypan blue exclusion. A 99% minimum cell viability is required.
- Suspensions are adjusted so 100 µL of 50% Matrigel solution + LN-229 cell suspension contains one million cells. Cells are inoculated s.c. into a hind leg per mouse. The mice are NOD.CB17-Prkdc or athymic BALB/C strain and 9-11 weeks old at the tine of xenotransplantation.
- Calipers are utilized for tumor monitoring, with 80-120 mm3 size tumors needed to initiate the study. Mice are sorted into cohorts and test compounds are administered following the schedule.
- Tumor measurements and mouse weights are recorded until tumor size limits are reached. Necropsies are performed for tumor removal, weights are logged and digital images captured.
- Choices for tissue collection include: submersion in RNA-later, snap freeze, isolate nucleic acids or prepare for histological analysis.
Get Instant Quote for
LN-229 Xenograft Model
The LN229 is a cell line derived from a human glioblastoma, this cell line is commonly used in preclinical research to study the biology of glioblastoma and to test new treatments using LN-229 xenograft model. This is important because it can help to determine whether a treatment is safe and effective before it is tested in humans. A xenograft is a transplant of cells or tissue from one species to another (in medicine, it is often also used to refer to a tissue graft from a human to a non-human specie). Xenotransplantation is the process of transplanting tissue or organs from one species to another. Xenograft animal models are commonly used to test the effectiveness of drugs against specific types of cancer. All clinically approved anticancer agents have been evaluated with conventional preclinical in vivo models. Xenograft studies can be highly complex, starting with the selection of the appropriate animal model, choice of tumorigenic cell line, administration method, dosing, analysis of tumor growth rates and tumor analysis (histology, mRNA and protein expression levels).
The dosing of the experimental compound of interest is initiated, for a staged study, when the mean tumor size reaches a specified volume (typically 50-100 mm3). Altogen Labs provides an array of laboratory services using over 90 standard Cell Line Derived Xenograft (CDX) models and over 30 PDX models. Researchers investigating the role of specific proteins or gene products in regulating tumor growth can benefit from development of protein overexpression (genetically engineered to ectopically express proteins, tumor suppressors, or oncogenes) and RNAi cell lines with long term gene silencing. Altogen Labs provides quantitative gene expression analysis of mRNA expression (qPCR) and protein expression analysis using the WES system (ProteinSimple).
Animal handling and maintenance at the Altogen Labs facility is IACUC-regulated and GLP-compliant. Following acclimatization to the vivarium environment, mice are sorted according to body mass. The animals are examined daily for tumor appearance and clinical signs. We provide detailed experimental procedures, health reports and data (all-inclusive report is provided to the client that includes methods, results, discussion and raw data along with statistical analysis). Additional services available include collection of tissue, histology, isolation of total protein or RNA and analysis of gene expression.
Following options are available for the LN-229 xenograft model:
- LN-229 Tumor Growth Delay (TGD; latency)
- LN-229 Tumor Growth Inhibition (TGI)
- Flexible dosing frequency and duration of dose administration
- Dosing route (intravenous, intratracheal, continuous infusion, intraperitoneal, intratumoral, oral gavage, topical, intramuscular, subcutaneous, intranasal; injections are performed using cutting-edge micro-injection techniques and pump-controlled IV injection)
- LN229 tumor immunohistochemistry (typically part of the preclinical Pharm/Tox studies)
- Alternative cell engraftment sites (orthotopic transplantation, tail vein injection and left ventricular injection for metastasis studies, injection into the mammary fat pad, intraperitoneal injection)
- Blood chemistry analysis (typically part of the preclinical Tox studies)
- Toxicity and survival (optional: performing a broad health observation program)
- Gross necropsies and histopathology (typically part of the preclinical toxicology studies)
- Positive control group employing cisplatin administered daily for the study duration
- Optional lipid distribution and metabolic assays
- Imaging studies: Fluorescence-based whole body imaging
Get Instant Quote for
LN-229 Xenograft Model
LN229 is a human glioblastoma cell line commonly used in brain cancer research. It was derived from a malignant glioblastoma tumor in a patient and is now widely used as a model system to study the biology of brain tumors and test potential cancer therapies. LN-229 cells have been extensively characterized and are known to harbor several genetic mutations, including mutations in the tumor suppressor gene p53 and the oncogene EGFR. These mutations are commonly found in glioblastoma tumors and are thought to contribute to tumor growth and resistance to therapy. LN229 cells are also notable for their ability to form tumors when injected into immunodeficient mice. This property makes them useful for studying the biology of glioblastoma tumors in vivo, and for testing potential cancer therapies in nonclinical models.
LN229 cells have been used in numerous xenograft studies to investigate the mechanisms of glioblastoma growth and metastasis, as well as to test the efficacy of various therapeutic strategies, including chemotherapy, radiation therapy, and immunotherapy. These studies have contributed to our understanding of the biology of glioblastoma and have helped to identify new targets for the development of more effective brain cancer treatments. Overall, LN229 is a valuable tool in brain cancer research, and its use has contributed to significant advances in our understanding of the disease and the development of new therapies.
