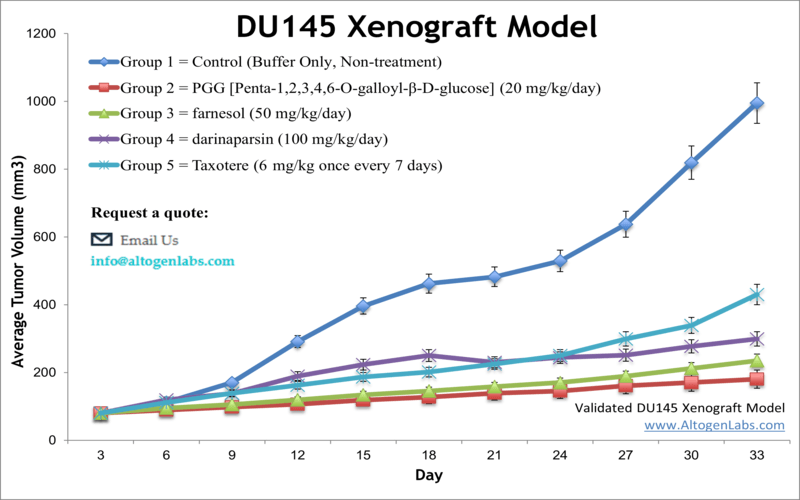
DU145 xenograft model
Prostate cancer is the most prevalent cancer in older males and is the second common malignancy in the Western world. Xenograft nonclinical studies can be conducted to find more complex treatment for patients with advanced stage disease. The DU-145 epithelial cell line was isolated from prostate carcinoma cells of a 69-year-old Caucasian male. DU-145 has microvilli, tonofilaments, and desmosomes. The DU-145 cell line is not hormone sensitive and fails to express prostate-specific antigen. Concurrent chemoradiotherapy (CCRT) has proven to be a potent tool used during the past decades. A 2014 study by Wang et al. published in Clinical and Translational Oncology examined the efficacy of combining radiation with low-dose docetaxel in the DU-145 prostate cancer xenograft model. The article indicates that CCRT with low-dose docetaxel blocks the growth of DU-145 prostate cancer xenografts in vivo by enhancing apoptosis and suppressing angiogenesis. This suggests CCRT with low-dose docetaxel could be a new treatment strategy for prostate cancer patients. A 2011 Journal of Cancer Prevention article (Lim et al.) used the DU145 to examine the prostate tumor microenvironment, specifically the interaction between tumor cells and THP-1 human monocytes. Results demonstrated that prostate cancer cells that were co-inoculated with human monocytes resulted in increased Ki67, VEGF and IL-8 which may promote tumor growth. In 2012 Cancer Biology released an article by Gilloteaux et al. charactarizing the morphology of DU145 xenografts in nude mice. They concluded that xenografts of DU145 display two primary carcinoma cell types: either actively replicating clear cells that grow into intraperitoneal tumors and are poorly differentiated or invasive basophilic cells that invade and replace peritoneal stroma of organs. A commonly observed phenotype with formed tumors were spheroid aggregates with pleiomorphic microvilli. Lastly, Mabuchi et al. (2017) released a study with Anticancer Research using the DU145 xenograft model in nude mice to evaluate the chemotherapeutic efficacy of docetaxel in prostate cancer. Results demonstrated a dose-dependency and various body weight side effects that were dependent on implantation approach. The DU-145 cell line (human prostate) is used to create the CDX (Cell Line Derived Xenograft) DU-145 xenograft mouse model. The DU-145 xenograft model enables studies of tumor growth inhibition by systemic viral agents, small molecules (farnesol, darinaparsin, taxotere) or siRNA/miRNA.
DU145 xenografts are mouse models of human prostate cancer that are created by implanting DU145 cells into immunodeficient mice. These models are commonly used in preclinical studies to evaluate the efficacy of potential therapeutic agents for prostate cancer and to investigate the biology of prostate cancer metastasis. DU145 xenografts have been extensively characterized and are known to exhibit many of the hallmarks of human prostate cancer, including androgen independence, invasiveness, and metastatic potential. These models have been used to study various aspects of prostate cancer biology, including the molecular and genetic mechanisms underlying cancer progression and the interactions between cancer cells and the tumor microenvironment.
Download Altogen Labs DU145 Xenograft Model PowerPoint Presentation: ![]()
Basic study design
- Cells are maintained under conditions of exponential growth.
- The DU145 cells are then collected from the flasks by trypsinization followed by determining viable cell counts using a trypan blue exclusion assay (minimum of 98% cell viability). Cell suspension concentration is adjusted to the appropriate density.
- Each mouse (athymic BALB/c (nu/nu), 10-12 weeks old) receives a single subcutaneous (s.c) injection into the flank of the hind leg. Each injection contains one million cells (in 100 µL) of the Matrigel-DU-145 cell suspension.
- Injection sites are palpated up to three times a week until tumors are established. Tumors are measured via digital calipers until an average size of 50-150 mm3 is reached.
- According to the treatment schedule, animals are randomized into the treatment cohorts. Administration of the test compound is performed.
- Tumors are continually measured daily while mouse weights are recorded 3 times a week.
- Animals are humanely euthanized as tumor size reaches 2,000 cu millimeters, or the predetermined size limit of the study.
- A necropsy and tissue collection is performed at the termination of the experiment. Tumors are excised and weighed, and then documented by digital imaging.
- Gross necropsies are performed to collect tissues for downstream analysis.
- The tumors/tissues can be submersed in RNAlater, snap frozen in LN2, prepared for histology or nucleic acid isolated for genetic analysis.
Get Instant Quote for
DU145 Xenograft Model
All clinically approved anti-cancer agents have been evaluated with conventional preclinical in vivo models. Altogen Labs provides an array of laboratory services using over 90 standard cell line-derived models and over 30 PDX models. Xenograft animal models are used to assess the effectiveness of drugs against specific types of cancer. New medicines are tested on staged tumor growths that have been engrafted via subcutaneous or orthotopic inoculation in an immunocompromised mouse or rat model. Xenograft studies can be highly complex, starting with the selection of the appropriate animal model, choice of tumorigenic cell line, administration method, dosing, analysis of tumor growth rates and tumor analysis (histology, mRNA and protein expression levels). Animal handling is regulated by IACUC and compliant to GLP guidelines. Following acclimatization to the vivarium environment, mice (or rats) are sorted according to body mass. The animals are examined daily for tumor appearance and clinical signs. We provide detailed experimental procedures, health reports and data (all-inclusive report is provided to the client that includes methods, results, discussion and raw data along with statistical analysis).
Following options are available for the DU145 xenograft model:
- DU-145 Tumor Growth Delay (TGD; latency)
- DU-145 Tumor Growth Inhibition (TGI)
- Dosing frequency and duration of dose administration
- Dosing route (intravenous, intratracheal, continuous infusion, intraperitoneal, intratumoral, oral gavage, topical, intramuscular, subcutaneous, intranasal, using cutting-edge micro-injection techniques and pump-controlled IV injection)
- DU-145 tumor immunohistochemistry
- Alternative cell engraftment sites (orthotopic transplantation, tail vein injection and left ventricular injection for metastasis studies, injection into the mammary fat pad, intraperitoneal injection)
- Blood chemistry analysis
- Toxicity and survival (optional: performing a broad health observation program)
- Gross necropsies and histopathology
- Positive control group employing standard chemotherapy drugs
