A549 xenograft model (subcutaneous and metastatic)
Lung cancer is the leading cause of cancer-related deaths worldwide. Non-small cell lung cancer (NSCLC) is responsible for nearly 85 percent of all lung cancer cases. Despite recent advances in therapeutic regimens, the mortality rate of NSCLC is still very high. The A549 hypotriploid epithelial cell line was derived from skin cells of a 58-year-old Caucasian male with pulmonary adenocarcinoma in 1972 by D. J. Giard. The A549 cell line is an excellent host for studies of lung cancer as well human infections related to the lungs. A549 is a well-characterized cellular model for allergies, asthma, and respiratory infections. A 2012 study by Harris et al used the A549 xenograft model to demonstrate that spontaneous metastases occur with this NSCLC lung cancer murine model as typically injections of primary xenografts is artificial metastasis; the group concluded that the A549 model when xenografted into mice liver (rather than flank) resulted in a gradual development metastatic spread. In 2013 Jakubowska et al. published data demonstrating that subcutaneous implantation in nude mice also results in pulmonary metastases in A549 xenografted mice. A 2015 study published in International Journal of Molecular Sciences investigated the antitumor and apoptotic effects of myricanol on the A549 xenograft in nude mice in vivo. The results indicate that myricanol suppresses tumor growth in vivo and induces inhibitory effects on the A549 xenograft model of lung adenocarcinoma. This mechanism is associated with apoptotic cell death by down-regulating Bcl-2, VEGF, HIF-1α, and survivin expressions and up-regulating Bax expression. Thus, myricanol could be a clinical candidate for the treatment of NSCLC. The 2015 Clinical Cancer Research study by Cai et al. used the A549 xenograft model to analyze the therapeutic potential of vasostatin, a potent angiogenesis inhibitor, delivered by the recombinant pseudotype adeno-associated virus 2/5 (rAAV2/5-VAS). Results showed that treatment with rAAV2/5-VAS led to tumor growth and metastasis suppression, validating this gene therapy as a potential NSCLC treatment. Xu and Prestwich published a 2010 in Cancer in which they engineered a 3-D tumor xenograft oaf A549 in order to demonstrate that BrP-LPA treatment results in tumor regression and growth and angiogenesis inhibition. BrP-LPA is an inhibitor of lysophosphatidic acid (LPA) biosynthesis and dually an agonist of the LPA receptor and so interferes with lysophospholipid signaling. The A549 cell line (human lung) is used to create the CDX (Cell Line Derived Xenograft) A549 xenograft mouse model. The A549 CDX model is the highly utilized xenograft lung cancer model for studying standard of care and novel therapeutics (i.e. erlotinib, gefitinib, lapatinib, paclitaxel) due to overexpression of EGFR and HER-2.
Download Altogen Labs A549 Xenograft Model PowerPoint Presentation: ![]()
Basic study design
- The cells used for injection are maintained in asceptic conditions and at exponential growth prior to cells injection (xenotransplantation).
- A549 cells are prepared by trypsinization and the percentage of viable cells are determined using flow cytometry, MTT, or trypan blue exclusion assay (98-99% cell viability required). Cell suspensions are adjusted to the appropriate cell density.
- Each mouse (athymic BALB/C or NOD/SCID, 10-12 weeks old) receive a subcutaneous injection in the hind leg. Each injection contains 100-120 microliters of one million cells with matrigel (A549 cell suspension).
- The injection sites are palpated three times weekly until tumors are established. Tumors are continually measured with digital calipers until they reach an average size of 75-150 mm3.
- Animals are then randomized into the necessary cohorts and administration of the test compound is performed following the experiment design schedule.
- Daily measurements of tumors are performed, while mouse weights are recorded 3 times weekly.
- At the end of the study, a necropsy is performed and tissues are collected as defined.
- All tumors are excised and weighed; tumors are documented by digital imaging.
- Histopathology and gross necropsies are performed on the study animals and tissues are collected for downstream analysis.
- The tissues and tumors can be snap frozen in LN2, stabilized in RNA-later reagent, and prepared for histology and/or gene expression analysis.
Metastatic Model
CDX models are mouse xenografts used in nonclinical therapeutic studies. However, as primary tumors proliferate they invade surrounding tissue, become circulatory, survive in circulation, implant in foreign parenchyma and proliferate in the distant tissue. This result leads to an extremely high percentage of death in cancer patients due to metastasis. Metastatic tumor mouse models are utilized to develop novel therapeutic agents that target metastasis (anti-metastatic therapeutics). To create a metastatic model, the cell line of interest is transfected with vectors containing green fluorescent protein (GFP) or luciferase. Maintained under antibiotic selection, only cells containing the integrated vector will survive. The new cell line clones are capable of stably expressing the gene of interest and are used in metastatic mouse model studies. Although each new cell line clone may contain its own inherent difficulties, the new cell line contains the ability to track internal tumor progression via bioluminescence (luciferase fluorescence after injecting luciferin) or fluorescence (GFP). Internal orthotopic and metastatic tumor growth (not palpable) can now be measured throughout the study, enabling a researcher to gain more insight and additional data in contrast to relying on end of study tumor weight measurements.
Case Study: U87-Luc Xenograft Model
An example of Altogen Labs utilizing a luciferase expressing cell line to monitor orthotopic tumor growth is exhibited below. The same ideology of tumor observation is incorporated in metastatic tumor models.
Luciferase expressing U87-luc cells were implanted and tumors allowed to grow. Tumor growth was monitored in a Night Owl (Berthold Technologies) imaging system 10 minutes after an intraperitoneal (IP) injection of the luciferin substrate. As seen in the example below, luciferase expression (measured as photons emitted) in the U87-luc model grants the researcher a visual image and quantifiable metric for orthotopic or metastatic tumor progression.
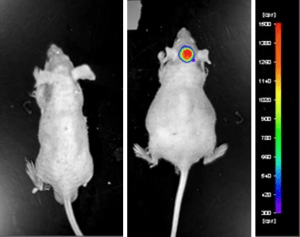
Figure 1. Luciferase expression in U87-luc orthotopic model. Control and implanted glioma mouse model fluorescence was analyzed 10 minutes after intraperitoneal luciferin injection.
View full details of the case study by clicking here.
Get Instant Quote for
A549 Xenograft Model
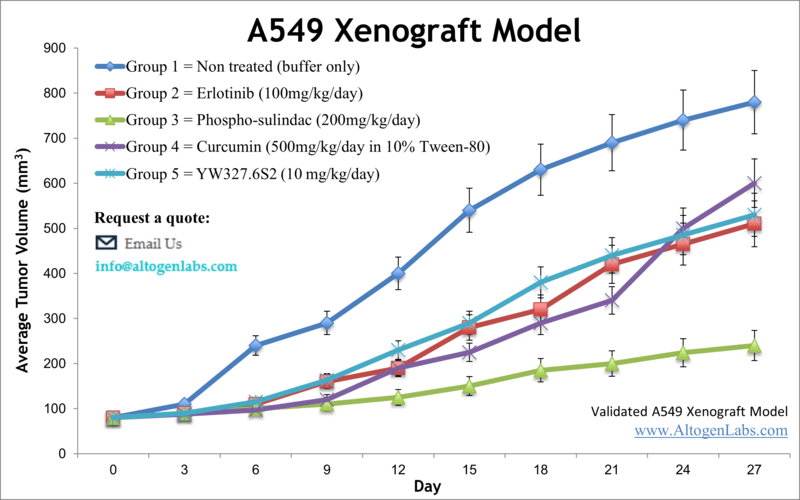
Xenograft animal models are used to assess the effectiveness of drugs against specific types of cancer. New medicines are tested on staged tumor growths that have been engrafted via subcutaneous or orthotopic inoculation in an immunocompromised mouse or rat model. All clinically approved anti-cancer agents have been evaluated with conventional preclinical in vivo models. Xenograft studies can be highly complex, starting with the selection of the appropriate animal model, choice of tumorigenic cell line, administration method, dosing, analysis of tumor growth rates and tumor analysis (histology, mRNA and protein expression levels). Altogen Labs provides an array of laboratory services using over 120 CDX and PDX models. Animal handling at the Altogen Labs facility is regulated by IACUC and compliant to GLP guidelines. We provide detailed experimental procedures, health reports and data (all-inclusive report is provided to the client that includes methods, results, discussion and raw data along with statistical analysis). Additional services available include collection of tissue, histology, isolation of total protein or RNA and analysis of gene expression.
Following options are available for the A549 xenograft model:
- A549 Tumor Growth Delay (TGD; latency)
- A549 Tumor Growth Inhibition (TGI)
- Dosing frequency and duration of dose administration
- Dosing route (intravenous, intratracheal, continuous infusion, intraperitoneal, intratumoral, oral gavage, topical, intramuscular, subcutaneous, intranasal, using cutting-edge micro-injection techniques and pump-controlled IV injection)
- Safety toxicology, ADME, A549 tumor immunohistochemistry, etc
- Alternative cell engraftment sites (orthotopic transplantation, tail vein injection and left ventricular injection for metastasis studies, injection into the mammary fat pad, intraperitoneal injection)
- Blood chemistry analysis, toxicity and survival
- Positive control group employing known chemotherapy compound
