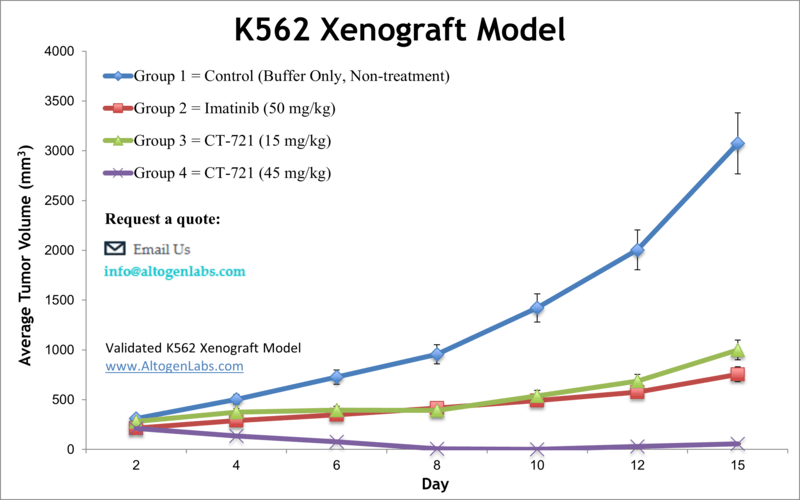
K562 xenograft model (subcutaneous and metastatic)
Leukemia is a malignancy of the blood cells that can be acute or chronic. Although leukemia mainly affects older adults, it is also the most prevalent malignancy in children younger than 15 years, as stated by NCI. Preclinical studies of cell-derived xenograft mouse models aid in finding new treatment regimens for leukemia patients. The K562 tumorigenic cell line was derived from the bone marrow of a 53-year-old female patient with chronic myelogenous leukemia (CML) and possesses lymphoblast morphology. In a 2001 study published in Proceedings of the National Academy of Sciences of the United States of America, the therapeutic efficacy of 12,13-desoxyepothilone B (dEpoB) was compared with paclitaxel, doxorubicin, and vinblastine using K562 xenografts in nude mice. According to the article, dEpoB is more effective than paclitaxel, doxorubicin, and vinblastine. However, during the evaluation of dEpoB and 12,13-desoxyepothilone F (dEpoF), dEpoF has proven to be more effective as it suppresses tumor growth and also results in tumor shrinkage in the K562 xenograft model. A 2015 Clinical Cancer Research article by Caruana et al. used the K562 model line to develop a whole-cell vaccine boost to chimeric antigen receptor (CAR)-redirected virus-specific cytotoxic T cells (CTLs); the vaccine was designed to express CMV-pp65, OX40L, iC9 and CD40L and antitumor effects of CAR-redirected cytomegalovirus-CTLs were successfully enhanced. Tian et al. (2015) used K562 cells to develop a HLA knockout strain as a method for expanding CD1d-restricted type-1 natural killer T cells (NKTs) on a clinical scale as these cells have shown to have antitumor activity but are often limited by their low numbers. The successful production of NKTs provides higher viability for the option for cancer immunotherapy using NKTs. Lastly, a 2009 study by Zhang et al. used the K562 xenograft model to test the efficiency of a transferrin (tf) receptor targeted lipopolyplex (LP) to deliver an antisense oligonucleotide (G3139) targeting Bcl-2. Results showed the successful downregulation of Bcl-1 in tf-LP-G3139 treated models as well as in vivo growth inhibition, thereby supporting the clinical potential for this therapeutic technique. The K562 cell line (human leukemia) is used for the creation of the CDX (Cell Line Derived Xenograft) K562 xenograft mouse model. The K562 xenograft model can be used to investigate the pivotal role of tissue inhibitors of metalloproteinases (TIMPs) for the treatment of leukemia. K562 xenograft models have also been used to study the molecular mechanisms underlying leukemia development and progression. For example, studies have shown that the overexpression of certain genes, such as Bcl-2 or c-Myc, can promote the growth of K562-derived tumors in vivo. These studies have provided valuable insights into the molecular pathways involved in leukemia pathogenesis and have identified potential targets for therapeutic intervention.
Download Altogen Labs K562 Xenograft Model PowerPoint Presentation: ![]()
Basic study design
1. K562 cells are maintained in suspension at exponential phase growth.
2. The cells are prepped for injection by determining the viable number of cells using trypan blue (minimum of 98% viability). Cell counts are adjusted to the appropriate density for injection.
3. Each mouse (10 to 12 weeks old; athymic BALB/C or NOD/SCID) receives single s.c. injections into the flank of a hind leg. Each injection contains one million cells of the K562 + matrigel suspension (vol = 100 microliters).
4. The injections are palpated until tumors are determined. Tumors are measured using calipers (digital) until an average size of 75-150 mm3 is reached.
5. Animals are randomized into appropriate groups . Administration of test material is performed according manufacturer recommendations.
6. Tumors are measured (daily) and mouse weights recorded (3 times weekly).
7. Animals are humanely euthanized when maximum tumor size is reached (or 2,000 cubic millimeters).
8. At termination of experiment, tumors are excised and weighed. All tumors are digitally imaged.
10. Necropsies are performed and all tissues collected as requested by client.
11. Tissues can be 1) snap frozen in LN2, 2) prepared for histology, or 3) nucleic acids isolated.
Metastatic Model
CDX models are mouse xenografts used in pre-clinical therapeutic studies. However, as primary tumors proliferate they invade surrounding tissue, become circulatory, survive in circulation, implant in foreign parenchyma and proliferate in the distant tissue. This result leads to an extremely high percentage of death in cancer patients due to metastasis. Metastatic tumor mouse models are utilized to develop novel therapeutic agents that target metastasis (anti-metastatic therapeutics).
To create a metastatic model, the cell line of interest is transfected with vectors containing green fluorescent protein (GFP) or luciferase. Maintained under antibiotic selection, only cells containing the integrated vector will survive. The new cell line clones are capable of stably expressing the gene of interest and are used in metastatic mouse model studies. Although each new cell line clone may contain its own inherent difficulties, the new cell line contains the ability to track internal tumor progression via bioluminescence (luciferase fluorescence after injecting luciferin) or fluorescence (GFP). Internal orthotopic and metastatic tumor growth (not palpable) can now be measured throughout the study, enabling a researcher to gain more insight and additional data in contrast to relying on end of study tumor weight measurements.
Case Study: U87-luc Xenograft Model
An example of Altogen Labs utilizing a luciferase expressing cell line to monitor orthotopic tumor growth is exhibited below. The same ideology of tumor observation is incorporated in metastatic tumor models.
Luciferase expressing U87-luc cells were implanted and tumors allowed to grow. Tumor growth was monitored in a Night Owl (Berthold Technologies) imaging system 10 minutes after an intraperitoneal (IP) injection of the luciferin substrate. As seen in the example below, luciferase expression (measured as photons emitted) in the U87-luc model grants the researcher a visual image and quantifiable metric for orthotopic or metastatic tumor progression.
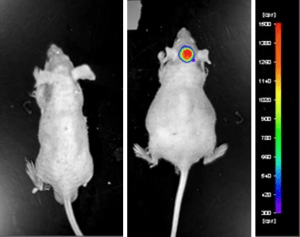
Figure 1. Luciferase expression in U87-luc orthotopic model. Control and implanted glioma mouse model fluorescence was analyzed 10 minutes after intraperitoneal luciferin injection.
View full details of the case study by clicking here.
Get Instant Quote for
K562 Xenograft Model
Xenograft animal models are used to assess the effectiveness of drugs against specific types of cancer. New medicines are tested on staged tumor growths that have been engrafted via subcutaneous or orthotopic inoculation in an immunocompromised mouse or rat model. All clinically approved anti-cancer agents have been evaluated with conventional preclinical in vivo models. Xenograft studies can be highly complex, starting with the selection of the appropriate animal model, choice of tumorigenic cell line, administration method, dosing, analysis of tumor growth rates and tumor analysis (histology, mRNA and protein expression levels).
Altogen Labs provides an array of laboratory services using over 90 standard CDX models and over 30 PDX models. Researchers investigating the role of specific proteins or gene products in regulating tumor growth can benefit from development of protein overexpression (genetically engineered to ectopically express proteins, tumor suppressors, or oncogenes) and RNAi cell lines with long term gene silencing. Altogen Labs provides quantitative gene expression analysis of mRNA expression (RT-PCR) and protein expression analysis using the WES system (ProteinSimple).
Following options are available for the K562 xenograft model:
- K562 Tumor Growth Delay (TGD; latency)
- K562 Tumor Growth Inhibition (TGI)
- Dosing frequency and duration of dose administration
- Dosing route (intravenous, intratracheal, continuous infusion, intraperitoneal, intratumoral, oral gavage, topical, intramuscular, subcutaneous, intranasal, using cutting-edge micro-injection techniques and pump-controlled IV injection)
- K562 tumor immunohistochemistry
- Alternative cell engraftment sites (orthotopic transplantation, tail vein injection and left ventricular injection for metastasis studies, injection into the mammary fat pad, intraperitoneal injection)
- Blood chemistry analysis
- Toxicity and survival (optional: performing a broad health observation program)
- Gross necropsies and histopathology
- Positive control group employing cyclophosphamide, at a dosage of 25-50 mg/kg
