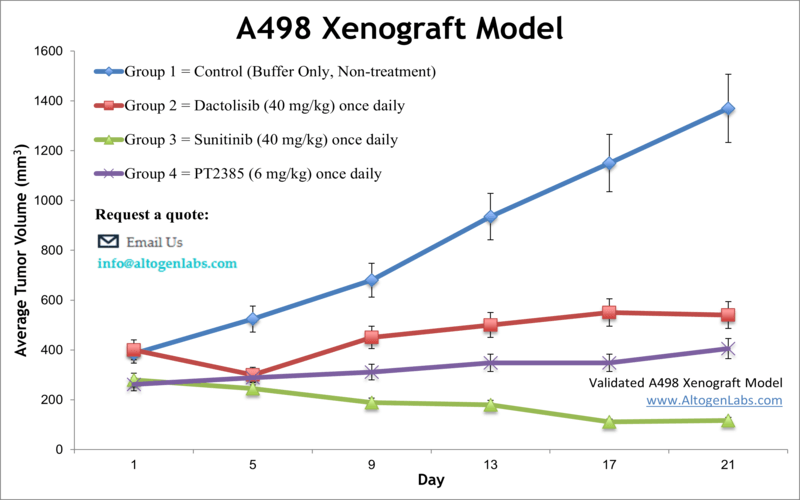
A498 xenograft model
The A498 cell line is a human kidney cancer cell line that was derived from the renal cell carcinoma of a patient in 1975. A498 cells are commonly used in cancer research to study the biology of kidney cancer and test new therapies. Renal cancer is responsible for nearly 63,000 new cases diagnosed annually with an average age at diagnosis of 64 years, as per the National Cancer Institute. The exact cause of renal cancer is unknown, and scientists often rely on preclinical research in the development of innovative kidney therapeutics. Despite the improvements in outcomes for patients with advanced RCC, the 5-year relative survival rate remains low, signaling that improved therapeutic regimens are still required. Xenograft studies have a significant impact on the development of cancer treatment. The A498 epithelial cell line was isolated from the kidney carcinoma of a 52-year-old male by D.J. Giard in 1973. The A498 cell line is tumorigenic in nude mice. A 2017 study in Cancer Science demonstrates with the A498 model line that simultaneous targeting of tumor cell growth and angiogenesis by a combination of lenvatinib and everolimus resulted in enhanced antitumor activity and led to tumor regression and that the inhibition of both VEGF and FGF signaling pathways by the combination proves its anti-angiogenic activity in the A498 RCC xenograft model. The 2008 study by Wu et al. used the A498 cell model to characterize the drug YC-1 ({3-(5′-hydroxy methyl-2′-furyl)-1-benzylindazole}) which was first discovered for its antiplatelet activity and was later shown to target HIF-1 pathways including VEGF and erythropoietin. Results from the study showed increased phosphorylation of JNK, cytotoxicity induced by YC-1 and an increase in apoptosis via mitochondria and caspase mediated pathways. In 2013 Fang et al. released a study using the A498 cells to test 3-hydroxy-3-methyl glutaryl coenzyme A (HMG-CoA) reductase inhibitors to improve renal cell carcinoma prognosis. Data demonstrated the ability of simvastatin to reduce proliferation, motility and in vivo tumor growth; results also suggest this mechanism is via inhibition of IL-6 induced phosphorylation of STAT3 AND JAK2 (AKT/mTOR and ERK were also downregulated) that results in reduction metastasis and promotion of apoptosis. A 2011 study (Zhang et al.) used A498 cells to demonstrate that the cytokine interleukin-22 (IL-22) suppresses proliferation of renal cell carcinoma via a STAT1 pathway and cell cycle arrest. It was previously thought that the mechanism was through apoptosis induction and upregulation of inflammatory cytokines; understanding drug mechanisms is important for the development of appropriate therapies. The A498 cell line (human kidney) is used to create the CDX (Cell Line Derived Xenograft) A498 xenograft mouse model. The A498 xenograft model is currently utilized in preclinical studies involving apoptosis thru activation of JNK pathway (e.g. YC-1), inhibitors targeting hypoxia-inducible factor (HIF; e.g. ELR510444) and anti-tumor efficacy (e.g. IL-22).
Download Altogen Labs A498 Xenograft Model PowerPoint Presentation: ![]()
Basic study design
- A498 cells are cultured in aseptic conditions growing at exponential phase. The cells are trypsinized and then viable cell counts are determined using trypan blue exclusion. The cell suspension is adjusted such that a 100-150 µL injection contains 1 x 106 cells (Matrigel + A498 cells).
- The mice, athymic BALB/C or NOD/SCID (10 to 12 weeks) receive single, subcutaneous injection in the hind leg. Manual palpation of injection sites aids in tumor establishment observation. At 80-120 mm3 tumor volume animals are sorted into study groups. Injections of the test compound follows the dose schedule.
- Daily tumor measurements and bi-weekly body weights are recorded. When tumor size approaches 2,000 mm3 or the predetermined size limit (per IACUC protocol) the mice are euthanized.
- Necropsies enable the collection of all tissues required for downstream analysis. Excised tumors are weighed and documented via digital imaging. All collected tissues are stored according to customer defined parameters: frozen, prepared for histology or nucleic acids isolated.
Get Instant Quote for
A498 Xenograft Model
Altogen Labs provides an array of laboratory services using over 90 standard CDX models and over 30 PDX models. Altogen Labs provides quantitative gene expression analysis of mRNA expression (qPCR) and protein expression analysis using the WES system (ProteinSimple). Animal handling and maintenance at the Altogen Labs facility is IACUC-regulated and GLP-compliant. Following acclimation to the vivarium environment, mice are sorted according to body mass. The animals are examined daily for tumor appearance and clinical signs. We provide detailed experimental procedures, health reports and data (all-inclusive report is provided to the client that includes methods, results, discussion and raw data along with statistical analysis). Additional services available include collection of tissue, histology, isolation of total protein or RNA and analysis of gene expression.
Following options are available for the A498 xenograft model:
- A498 Tumor Growth Delay (TGD; latency)
- A498 Tumor Growth Inhibition (TGI)
- A498 tumor immunohistochemistry
- Blood chemistry analysis
- Toxicity and survival (optional: performing a broad health observation program)
- Gross necropsies and histopathology
- Lipid distribution and metabolic assays
- Imaging studies: Fluorescence-based whole body imaging, MRI
