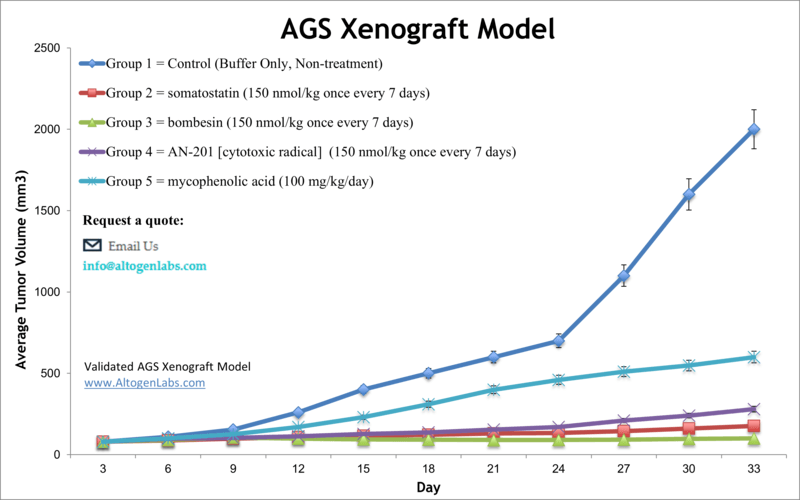
AGS xenograft model
Gastric cancer is the third primary cause of death from cancer worldwide. It is often diagnosed at an advanced stage with local invasion or metastasis. Due to the lack of early symptoms, it is usually hard to detect and difficult to cure. Xenograft murine models aid in studying gastric tumor growth and development aiming to find effective therapies. The AGS epithelial tumorigenic cell line was initially derived from the stomach tissue of a 54-year-old Caucasian female with gastric adenocarcinoma and is a reasonable host for studies related to different forms of stomach cancer. A 2013 AGS xenograft study published in Oncology Reports, investigated the effect of shRNA targeting autotaxin on the migratory and invasive capability of AGS gastric cancer cells. The article indicates that autotaxin could be a new molecular target for gastric cancer treatment. Another study that used the AGS xenograft model is Shin et al. (2016) which demonstrated that the phytosterol beta-sitosterol (BS) induces cell death in human gastric adenocarcinoma via apoptosis induction and activation of tumor suppressors including PTEN and APMK. In their 2012 study Li et al. used AGS cells to study the role of protocadherin 10 (PCDH10) hypermethylation in human gastric carcinoma. They found that PCDH10 exhibited reduced expression that correlated to hypermethylation in gastric cancer and that demethylation was correlated with restored expression and tumor growth suppression, implicating PCDH10 as a tumor suppressor gene (TSG) and potential therapeutic target. Lastly, Barati et al. released a 2018 study characterizing AGS xenografts in athymic nude mice as an appropriate research model of gastric adenocarcinoma; growth kinetics showed a 41 day mean doubling time and immunohistochemistry (IHC) of stomach neoplasms revealed model-specific pathology including specific p53 mutations and high CK8 expression and angiogenesis. The AGS cell line is routinely used to create the CDX (Cell Line Derived Xenograft) AGS xenograft mouse model. Due to cell surface expression of EGFR, the AGS model enables the study of anti-EGFR therapeutics such as cetuximab or carboplatin.
Download Altogen Labs AGS Xenograft Model PowerPoint Presentation: ![]()
Basic study design
- AGS cells used for injection are maintained under aseptic conditions and at exponential growth prior to injection
- AGS cells are prepared for injection using trypsin-EDTA and determining viable cell counts using a trypan blue assay (98% cell viability required). Cell suspension concentration is adjusted to the appropriate density
- Each mouse (NOD/SCID or athymic BALB/C; 10-12 weeks old) receives a hind leg subcutaneous injection of one million cells. Each injection contains a volume of 100 µl of the AGS cell suspension with Matrigel.
- Three times a week injection sites are palpated until tumors are established. Tumors are measured with digital calipers.
- Upon reaching an average size of 50-150 mm3, animals are randomized into appropriate treatment cohorts and the compound of interest is administered according to the established treatment schedule.
- Daily tumor measurements are taken and mouse weights are recorded 3 times weekly
- When tumor size reaches 2,000 cu millimeters (or IACUC-predetermined size limit) animals are euthanized.
- Animal necropsy is performed as defined for termination of experiment.
- Excised tumors are weighed and documented with digital imaging.
- Gross necropsies are performed; specified tissues are collected for downstream analysis.
- Tumors and tissues are snap frozen in LN2, stabilized in RNAlater reagent, prepared for gene expression analysis or histology.
Get Instant Quote for
AGS Xenograft Model
Xenograft animal models are used to assess the effectiveness of drugs against specific types of cancer. New medicines are tested on staged tumor growths that have been engrafted via subcutaneous or orthotopic inoculation in an immunocompromised mouse or rat model. All clinically approved anti-cancer agents have been evaluated with conventional preclinical in vivo models. Xenograft studies can be highly complex, starting with the selection of the appropriate animal model, choice of tumorigenic cell line, administration method, dosing, analysis of tumor growth rates and tumor analysis (histology, mRNA and protein expression levels).
Altogen Labs provides an array of laboratory services using over 30 standard Cell Line Derived Xenograft (CDX) models and over 20 PDX models. Researchers investigating the role of specific proteins or gene products in regulating tumor growth can benefit from development of protein overexpression (genetically engineered to ectopically express proteins, tumor suppressors, or oncogenes) and RNAi cell lines with long term gene silencing. Altogen Labs provides quantitative gene expression analysis of mRNA expression (RT-PCR) and protein expression analysis using the WES system (ProteinSimple).
Animal handling and maintenance at the Altogen Labs facility is IACUC-regulated and GLP-compliant. Following acclimation to the vivarium environment, mice are sorted according to body mass. The animals are examined daily for tumor appearance and clinical signs. We provide detailed experimental procedures, health reports and data (all-inclusive report is provided to the client that includes methods, results, discussion and raw data along with statistical analysis). Additional services available include collection of tissue, histology, isolation of total protein or RNA and analysis of gene expression.
Following options are available for the AGS xenograft model:
- AGS Tumor Growth Delay (TGD; latency)
- AGS Tumor Growth Inhibition (TGI)
- Dosing frequency and duration of dose administration
- Dosing route (intravenous, intratracheal, continuous infusion, intraperitoneal, intratumoral, oral gavage, topical, intramuscular, subcutaneous, intranasal, using cutting-edge micro-injection techniques and pump-controlled IV injection)
- AGS tumor immunohistochemistry
- Alternative cell engraftment sites (orthotopic transplantation, tail vein injection and left ventricular injection for metastasis studies, injection into the mammary fat pad, intraperitoneal injection)
- Blood chemistry analysis
- Toxicity and survival (optional: performing a broad health observation program)
- Gross necropsies and histopathology
- Positive control group employing cyclophosphamide, at a dosage of 50 mg/kg administered by intramuscular injection to the control group daily for the study duration
- Lipid distribution and metabolic assays
- Imaging studies: Fluorescence-based whole body imaging, MRI
