DLD-1 xenograft model
Colorectal cancer cell lines are extensively utilized nonclinical model systems, including the DLD1 cell line established from the tumorigenic epithelial tissue obtained from a male patient with colorectal adenocarcinoma by D.L. Dexter in 1979. In addition to being negative for ros, abl, and src oncogenes, the DLD-1 cell line is positive for myc, myb, ras, fos, sis, and p53 oncogenes. Also, DLD-1 cells express carcinoembryonic antigen (CEA) and colon antigen 3. The DLD-1 human colorectal cell line displays epithelial properties, including a cobblestone cell shape. Besides, DLD-1 expresses a truncated adenomatous polyposis coli (APC) protein, a tumor suppressor gene that plays a critical role in cell growth, as indicated in a 2016 article published in Biochemistry and Biophysics Reports. As one of the most commonly employed colon cancer cell lines, the DLD-1 cell line is involved in many medical and biomedical research applications related to colon cancer. One example is the 2015 study (Chen et al.) who used the DLD-1 xenograft model to study the oncogenic receptor tyrosine kinase (RTK) KIT in colorectal cancer (CRC); results indicated that KIT has a role in tumor growth promotion via autocrine or paracrine signaling. This provides a target for patients with KIT-expressing CRC. A 2008 study (Rhee et al.) used the DLD-1 xenograft model to study the role of toll-like receptor (TLR) signaling pathways, potential immunotherapy targets, in CRC. Results indicated that innate immunity is mediated by flagellin via TLR5 signaling which could be clinically relevant as a specific immunotherapy target. The last example for DLD-1 model usage in research is the 2012 study (Caysa et al.) which reported the development of a non-invasive method for imaging in vivo xenograft tumor growth and subsequent response to chemotherapy via multispectral fluorescence. DLD-1 cells are used to create the xenograft mouse model that enables studies on anti-EGFR therapeutics, such as IMC-C225, in combination with other conventional chemotherapies. The DLD1 cell line is known for its ability to form tumors in immunocompromised mice and has been widely used as a model system for studying various aspects of colorectal cancer biology.
DLD1 Xenograft Model: Subcutaneous And Orthotopic Tumor Model: ![]()
Download Altogen Labs DLD1 Xenograft Model PowerPoint Presentation: ![]()
Subcutaneous Xenografts of DLD-1 in Colorectal Cancer Research
Subcutaneous xenograft transplantation is one of the most widely utilized methodologies in preclinical oncology for evaluating tumor growth kinetics, therapeutic efficacy, and molecular responses in vivo. This approach involves the implantation of human cancer cells into the subcutaneous tissue of immunocompromised mice, providing a reproducible and accessible platform for monitoring tumor development and response to treatment. The DLD-1 colorectal adenocarcinoma cell line, derived from a Dukes’ type C colon tumor, is particularly well-suited for subcutaneous xenograft studies due to its stable tumorigenicity, well-defined genetic profile, and responsiveness to chemotherapeutic and targeted agents. As a microsatellite stable (MSS) cell line harboring oncogenic KRAS (G13D), TP53 (R273H), and APC mutations, DLD-1 provides a clinically relevant model for investigating tumor biology within the chromosomal instability (CIN) subtype of colorectal cancer. Numerous studies have employed subcutaneous DLD-1 xenografts to investigate resistance mechanisms to standard-of-care therapies such as 5-fluorouracil and oxaliplatin, as well as to evaluate novel targeted therapies including MEK, PARP, and ATR inhibitors. The model has also been instrumental in validating findings from CRISPR/Cas9-edited isogenic cell lines, particularly in delineating the contributions of oncogenic KRAS to drug sensitivity and tumor progression. The ease of tumor monitoring through external caliper measurements allows for high-throughput comparisons of treatment arms and longitudinal assessment of tumor growth inhibition. While subcutaneous models do not fully recapitulate the tumor microenvironment or immune interactions present in orthotopic settings, they remain a cornerstone of drug development pipelines due to their scalability and experimental tractability. Recent studies have begun to explore the integration of molecular endpoints such as transcriptomics, proteomics, and epigenetic profiling to enhance the translational relevance of subcutaneous xenografts. Emerging interest in the regulatory functions of long non-coding RNAs and their roles in chemoresistance underscore the utility of the DLD-1 model in dissecting post-transcriptional regulatory networks within an in vivo context. When implemented with robust experimental design and complemented by orthogonal molecular analyses, subcutaneous transplantation of DLD-1 cells offers a powerful and versatile approach for advancing colorectal cancer research.
Orthotopic Modeling of DLD-1 Colorectal Tumors
Orthotopic xenograft transplantation represents a critical advancement in preclinical modeling by enabling the implantation of human tumor cells into the organ of origin, thereby preserving essential aspects of the native tumor microenvironment, including stromal architecture, vascularization, and organ-specific interactions. In colorectal cancer research, orthotopic models have proven particularly valuable for studying tumor growth dynamics, local invasion, and the early steps of metastasis in a biologically relevant context. The DLD-1 colorectal adenocarcinoma cell line, originally derived from a Dukes’ type C tumor, has been successfully utilized in orthotopic implantation studies, despite its more common use in subcutaneous models. These orthotopic applications involve surgical implantation of DLD-1 cells into the cecal or colonic wall of immunocompromised mice, allowing for anatomically accurate tumor development and more predictive evaluations of therapeutic efficacy. Bioluminescent variants of DLD-1, such as DLD-1-luc, have facilitated longitudinal, non-invasive tracking of tumor growth and progression, enhancing the utility of this model in therapeutic studies. However, it is noteworthy that DLD-1 cells exhibit limited spontaneous distant metastatic potential in orthotopic settings, likely due to their low expression of pro-metastatic factors such as Fascin1. This characteristic, while limiting for metastasis-focused investigations, makes the model particularly suitable for evaluating localized tumor growth, stromal interactions, and responses to locoregional therapies. Incorporating orthotopic DLD-1 models into experimental pipelines allows researchers to interrogate drug efficacy within a more physiologically relevant microenvironment, offering a refined preclinical platform that bridges the gap between in vitro assays and clinical application.
Targeting Feedback Resistance in DLD-1 Colorectal Carcinoma
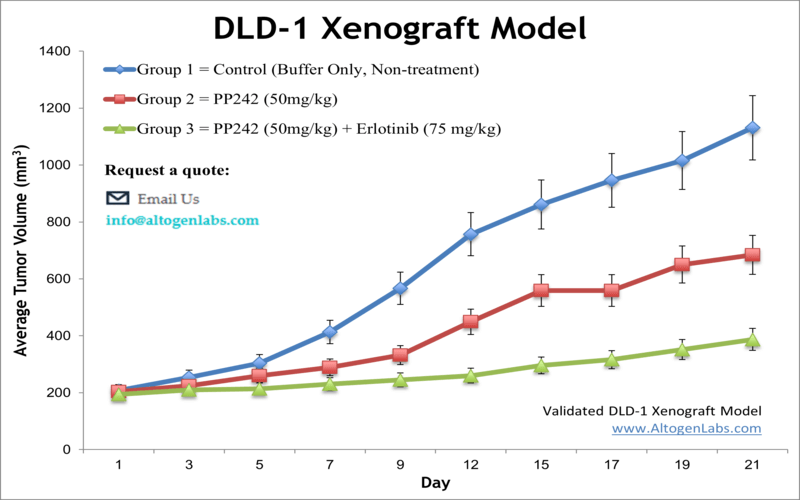
In a study published in PLoS One journal by Wang et al., the therapeutic potential of combining the mTOR kinase inhibitor PP242 with the EGFR inhibitor erlotinib was evaluated in colorectal carcinoma, with a particular focus on the DLD-1 cell line. The authors demonstrated that PP242 alone produces only transient inhibition of mTORC1 and mTORC2, as evidenced by temporary suppression of S6 and AKT (Ser473) phosphorylation followed by a rebound in activity. This recovery was linked to increased EGFR phosphorylation, occurring through a PI3K-independent pathway. When erlotinib was added, the combination sustained suppression of both mTOR complexes, reduced DLD-1 cell viability, inhibited colony formation, and triggered apoptosis, as shown by caspase-3, DFF45, and PARP cleavage. In vivo, subcutaneous DLD-1 xenografts treated with the combination exhibited significantly reduced tumor growth compared to those treated with PP242 alone or vehicle, reinforcing the synergistic effects of dual pathway inhibition. The data presented by Wang et al. support the conclusion that EGFR activation undermines the efficacy of second-generation mTOR inhibitors when used as monotherapy, and that co-targeting EGFR and mTOR enhances antitumor effects. The experimental design was comprehensive, employing Western blotting, RTK phosphorylation arrays, apoptosis assays, and xenograft models, although the small sample size of the animal study (n=4 per group) and omission of an erlotinib-only control group in vivo limit interpretive breadth. Nevertheless, the study offers valuable insights into the resistance mechanisms that compromise mTOR-targeted therapies. These findings position DLD-1 as a relevant preclinical model for evaluating combination strategies in KRAS-mutant, EGFR-expressing colorectal cancers. Future investigations should focus on identifying the downstream mediators of EGFR-driven mTORC2 reactivation and testing combination therapies in immune-competent or humanized models to further bridge the gap between preclinical and clinical research.
Dual Suppression of WNT and AKT Oncogenic Signaling in DLD-1 Cells
The combination of MM-129, a 1,2,4-triazine derivative, and indoximod, a kynurenine pathway inhibitor, exhibits significant antitumor activity in colorectal cancer models, with pronounced effects in DLD-1 cells. These cells, which carry activating mutations in KRAS and inactivating mutations in APC, display constitutive activation of the PI3K/AKT and WNT signaling pathways. Co-treatment disrupts these oncogenic cascades by downregulating AKT expression, reducing mitochondrial membrane potential, promoting phosphatidylserine externalization, and activating apoptotic markers including caspase-3/7, -8, and -10. Zebrafish xenograft models confirm these effects, with DLD-1-derived tumors showing a greater reduction in tumor burden and apoptotic signaling than HT-29. MM-129 drives the bulk of antiproliferative activity, while indoximod enhances cell death, likely through modulation of immunoregulatory and metabolic pathways. Notably, indoximod downregulates IDO1, a key enzyme in immune tolerance and epithelial-to-mesenchymal transition, although MM-129 alone does not affect IDO1 levels. A consistent pattern of synergistic action emerges, where MM-129 targets survival and proliferation, and indoximod complements these effects by interfering with immune evasion mechanisms. DLD-1 cells respond more robustly than HT-29, likely due to their distinct oncogene expression profile, including elevated COX-2, GRP78, and IDO1. The study employs a comprehensive methodology including flow cytometry, gene expression analysis, and zebrafish xenograft validation, though limitations remain in terms of long-term toxicity and immune system modeling in non-mammalian hosts. The findings underscore the therapeutic potential of dual-pathway targeting in colorectal cancer and validate the DLD-1 cell line as a relevant preclinical model for tumors with activated AKT and WNT signaling. Future research should prioritize mammalian validation, immune profiling, and exploration of pharmacodynamic interactions, with a focus on translating this drug combination into therapeutic strategies for colorectal tumors exhibiting the DLD-1 molecular phenotype.
Get Instant Quote for
DLD-1 Xenograft Model
DLD1 Xenograft Model: Subcutaneous And Orthotopic Tumor Model: ![]()
Basic study design
1. Until collection cells are maintained under exponential growth.
2. DLD-1 cells are prepared for injection via trypsinization. Viability of cells is determined using a trypan blue exclusion test (required 98-99% viability). The cell suspension is then adjusted to the appropriate density.
3. All mice (athymic BALB/C or NOD/SCID, 10-12 weeks old) receive a single subcutaneous injection into the hind leg, containing one million cells (100 µL of Matrigel/DLD-1 cell suspension).
4. All injection sites are palpated up to three times weekly until tumors are established. Tumors are then measured utilizing digital calipers; average size of 50-150 mm3.
5. Animals are randomized into treatment cohorts; administration of the compound of interest is performed following the treatment schedule.
6. Tumors are measured daily; mouse weights are recorded 3 times weekly.
7. Animals are euthanized as tumor size reaches 2,000 sq millimeters (or at the predetermined size limit for the study per IACUC protocol).
8. Final necropsy and tissue collection are performed as agreed upon for termination of experiment.
9. Tumors are removed and weighed; tumors are documented by digital imaging.
10. Gross necropsies are performed; tissues are collected for downstream analysis.
11. Tumors and tissues are prepared for histology, snap frozen in LN2 or stabilized for gene expression analysis.
Altogen Labs provides an array of laboratory services using over 90 standard Cell Line Derived Xenograft (CDX) models and over 30 PDX models. Researchers investigating the role of specific proteins or gene products in regulating tumor growth can benefit from development of protein overexpression (genetically engineered to ectopically express proteins, tumor suppressors, or oncogenes) and RNAi cell lines with long term gene silencing. Altogen Labs provides quantitative gene expression analysis of mRNA expression (RT-PCR) and protein expression analysis using the WES system (ProteinSimple).
Animal handling and maintenance at the Altogen Labs facility is IACUC-regulated and GLP-compliant. Following acclimatization to the vivarium environment, mice are sorted according to body mass. The animals are examined daily for tumor appearance and clinical signs. Additional services available include collection of tissue, histology, isolation of total protein or RNA and analysis of gene expression.
