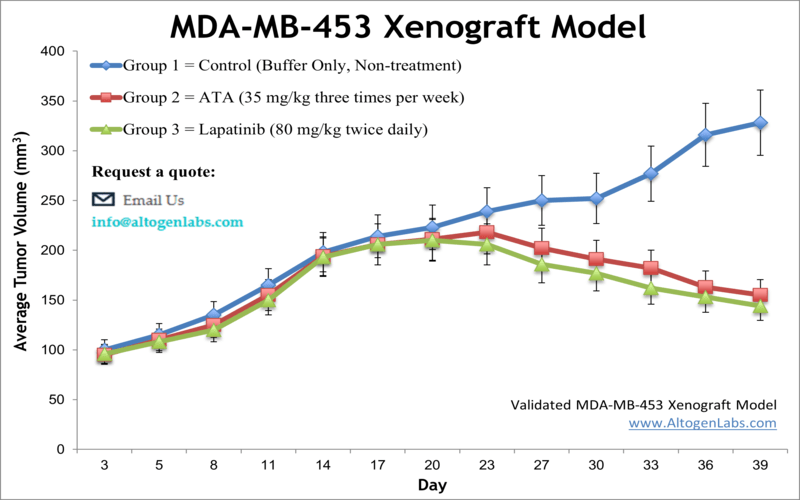
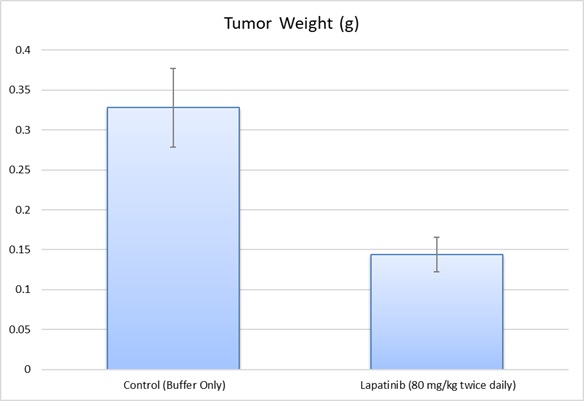
MDA-MB-453 xenograft model
Breast cancer contributes to over 458,000 deaths annually and 1.4 million newly diagnosed cases worldwide, as per the American Cancer Society. Xenograft murine models accurately mimic human tumor aiming to predict a patient’s response to chemotherapy. The MDA-MB-435 epithelial cell line was isolated in 1976 from a pericardial effusion of a 48-year old Caucasian female with metastatic breast carcinoma by Cailleau at M. D. Anderson Hospital and Tumor Institute, according to a 1980 study in Cancer Research. These cells are not tumorigenic and overexpress fibroblast growth factor (FGF). Also, MDA-MB-453 cells have an active glycerol 3-phosphate shuttle and express high amounts of functional androgen receptor (AR).They are not quite a triple negative breast cancer (TNBC) as sometimes reported in that they are estrogen receptor-alpha negative and progesterone receptor negative however they show Her-2/neu protein activity (Vranic et al., 2011). Studies that have used MDA-MB-453 cells include O-Brien et al. (2014) who published data supporting the use for combination therapy of PI3K/mTOR inhibition with tratsuzumab (a common chemotherapy drug for treating HER2+ cancers) for tratsuzumab-resistant breast cancer. Naderi et al. (2011) released a study demonstrating a synergistic effect between AR and extracellular signal-regulated kinase (ERK) inhibitors that also overcomes trastuzumab resistance that was verified in vitro and in vivo; this is particularly relevant for ER-/AR+ breast cancers. Finally, a 2011 Cancer Cell study (Ni et al.) studied endocrine therapies for breast cancer and used MDA-MB-453 for their ER-/AR+/HER2+ profile; their results deciphered the AR contribution to breast cancer as an activator of Wnt and HER pathways thereby stimulating tumor growth. The MDA-MB-453 xenograft model exhibits tumor growth inhibition from small molecules (e.g. enzalutamide, flutamide, CI-1040, DHT).
MDA-MB-453 Orthotopic And Metastatic Xenograft Model: Download ![]()
Download Altogen Labs MDA-MB-453 Xenograft Model PowerPoint Presentation: ![]()
The Orthotopic MDA-MB-453 Breast Cancer Model
The orthotopic MDA-MB-453 xenograft model is a valuable tool for studying molecular apocrine breast cancer, a subtype characterized by androgen receptor positivity and the absence of estrogen and progesterone receptors. In this model, MDA-MB-453 cells are implanted into the mammary fat pad of immunocompromised mice, providing a tumor microenvironment that closely mimics human breast cancer. Orthotopic implantation allows for the evaluation of tumor growth kinetics, therapeutic responses, and tumor-stroma interactions under physiologically relevant conditions. Studies utilizing this model have demonstrated its utility in assessing the efficacy of targeted therapies, including androgen receptor inhibitors and lipid-based drug formulations. Unlike more aggressive triple-negative breast cancer models, MDA-MB-453 tumors tend to exhibit moderate growth rates and lower metastatic potential. However, they provide an excellent platform for investigating the role of androgen signaling and alternative oncogenic pathways in breast cancer progression. The model also enables non-invasive imaging techniques and longitudinal studies, offering insights into tumor evolution and treatment resistance.
Metastatic MDA-MB-453 Xenograft Model
The metastatic MDA-MB-453 xenograft model is used to study the dissemination and colonization patterns of molecular apocrine breast cancer cells. While MDA-MB-453 tumors exhibit lower metastatic potential compared to highly aggressive triple-negative breast cancer models, they have been shown to spread under specific experimental conditions. Metastasis can be induced through orthotopic implantation followed by spontaneous dissemination, or by direct injection into circulation via the tail vein or left ventricle to model hematogenous spread. Studies suggest that MDA-MB-453 cells preferentially colonize the lungs, though metastatic efficiency is lower than that of basal-like breast cancer cell lines. This model enables researchers to investigate the role of androgen receptor signaling, tumor dormancy, and microenvironmental factors in metastatic progression. Additionally, it serves as a platform for testing anti-metastatic therapies, including androgen receptor antagonists and MEK inhibitors.
Get Instant Quote for
MDA-MB-453 Xenograft Model
MDA-MB-453 Xenografts Reveal the Therapeutic Promise of Acetyltanshinone IIA
A study conducted by Guerram M, et al., published by Oncotarget journal explores the anticancer potential of Acetyltanshinone IIA (ATA) in HER2-overexpressing breast cancer, with a focus on the MDA-MB-453 cell line. ATA, derived from tanshinone IIA, effectively inhibited the growth of MDA-MB-453 cells, inducing S-phase cell cycle arrest and apoptosis. Mechanistically, ATA downregulated HER2 and EGFR receptor tyrosine kinases, leading to the inhibition of downstream pro-survival signaling pathways. Additionally, ATA activated AMP-activated protein kinase (AMPK), suppressing lipid and protein biosynthesis, both essential for cancer cell proliferation. In a xenograft model using MDA-MB-453 tumors, intraperitoneal administration of ATA significantly reduced tumor size without toxic side effects. The study also demonstrated that ATA-induced oxidative and endoplasmic reticulum (ER) stress, contributing to cancer cell death. Furthermore, ATA inhibited angiogenesis and tumor cell migration, suggesting its potential role in preventing metastasis. Overall, these findings highlight MDA-MB-453 as a valuable model for studying ATA’s anti-cancer mechanisms and support its potential as a therapeutic agent for HER2-positive breast cancer.
Androgen Receptor Mutation in MDA-MB-453 Cells Alters Breast Cancer Signaling
Another study by Moore NL, et al., published by Endocrine-Related Cancer journal investigates the role of the MDA-MB-453 breast cancer cell line as a model for molecular apocrine breast cancer, a subtype characterized by androgen receptor (AR) signaling. Researchers identified a mutation in the AR gene of MDA-MB-453 cells, a G-T transversion in exon 7, leading to a Q865H amino acid substitution in the ligand-binding domain. This mutation resulted in reduced sensitivity to androgens such as dihydrotestosterone (DHT) and the progestin medroxyprogesterone acetate (MPA), altering AR transactivation potential. Functional analyses revealed that DHT and MPA activated distinct transcriptional programs, with DHT-associated genes resembling estrogen-responsive transcripts and engaging the Wnt signaling pathway, which promotes cell proliferation. In contrast, MPA regulated genes involved in cell cycle control and apoptosis, which may explain its inhibitory effects on MDA-MB-453 proliferation. Molecular modeling and ligand binding studies demonstrated that the Q865H mutation affects AR structural stability and intramolecular interactions. The findings highlight the necessity of studying alternative models of molecular apocrine breast cancer to fully understand AR’s role and assess its potential as a therapeutic target.
Key Oncogenic Features of MDA-MB-453 Breast Cancer Cells
The MDA-MB-453 breast cancer cell line is characterized by several oncogenic alterations that drive its aggressive phenotype. It exhibits a mutation in the K-RAS gene (Gly13Asp), leading to constitutive activation of the MAPK/ERK signaling pathway, which promotes rapid proliferation. The cell line also lacks the tumor suppressor p16INK4A due to a deletion at the 9p21 locus, resulting in deregulation of the cell cycle through a p16-/pRb-/cyclin D1+ phenotype. This alteration contributes to excessive growth rates, as indicated by a high S-phase fraction. Additionally, while MDA-MB-453 is classified as an androgen receptor (AR)-positive, estrogen receptor (ER)-negative model, it lacks EGFR and HER2 gene amplifications, distinguishing it from many apocrine carcinomas. Despite its molecular apocrine classification, key differences in oncogenic pathways compared to patient tumors raise concerns about its suitability as a universal model for apocrine breast cancer.
Basic study design
- MDA-MB-453 cells are trypsinized and total cell viability is determined
- After adjusting the cell suspension concentration to be one million cells per 100 µL (Matrigel w/ MDA-MB-453), 10 to 12 week old mice (athymic BALB/C or NOD/SCID) implanted with cells.
- All injection sites are observed and palpated until tumors are established.
- Randomization into the client determined treatment groups and administration of client supplied test compound is performed. Daily tumor measurements and mouse weights (2-3 times weekly) are logged.
- At the end of the study, tissues are collected and can be snap frozen in liquid N2, prepared for histological analysis or nucleic acids isolated.
Xenograft animal models are used to assess the effectiveness of drugs against specific types of cancer. New medicines are tested on staged tumor growths that have been engrafted via subcutaneous or orthotopic inoculation in an immunocompromised mouse or rat model. All clinically approved anti-cancer agents have been evaluated with conventional preclinical in vivo models. Xenograft studies can be highly complex, starting with the selection of the appropriate animal model, choice of tumorigenic cell line, administration method, dosing, analysis of tumor growth rates and tumor analysis (histology, mRNA and protein expression levels).
Animal handling and maintenance at the Altogen Labs facility is IACUC-regulated and GLP-compliant. Following acclimation to the vivarium environment, mice are sorted according to body mass. The animals are examined daily for tumor appearance and clinical signs. We provide detailed experimental procedures, health reports and data (all-inclusive report is provided to the client that includes methods, results, discussion and raw data along with statistical analysis). Additional services available include collection of tissue, histology, isolation of total protein or RNA and analysis of gene expression. Our animal facilities have the flexibility to use specialized food or water systems for inducible gene expression systems.
Following options are available for the MDA-MB-453 xenograft model:
- MDA-MB-453 Tumor Growth Delay (TGD; latency)
- MDA-MB-453 Tumor Growth Inhibition (TGI)
- Dosing frequency and duration of dose administration
- Dosing route (intravenous, intratracheal, continuous infusion, intraperitoneal, intratumoral, oral gavage, topical, intramuscular, subcutaneous, intranasal, using cutting-edge micro-injection techniques and pump-controlled IV injection)
- MDA-MB-453 tumor immunohistochemistry
- Alternative cell engraftment sites (orthotopic transplantation, tail vein injection and left ventricular injection for metastasis studies, injection into the mammary fat pad, intraperitoneal injection)
- Blood chemistry analysis
- Toxicity and survival (optional: performing a broad health observation program)
- Gross necropsies and histopathology
- Positive control group employing cyclophosphamide, at a dosage of 50 mg/kg administered by intramuscular injection to the control group daily for the study duration
- Imaging studies: Fluorescence-based whole body imaging
MDA-MB-453 Orthotopic And Metastatic Xenograft Model: Download ![]()
