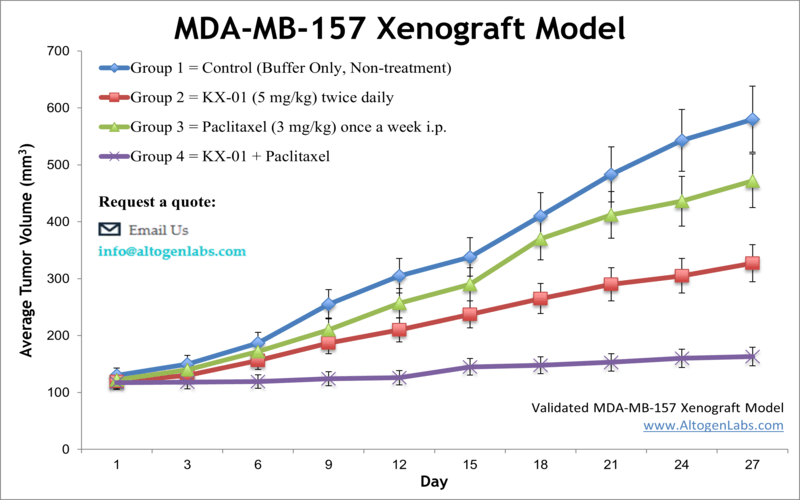
MDA-MB-157 xenograft model
Metastatic breast cancer is considered the leading cause of death in patients diagnosed with breast cancer. Preclinical xenograft studies can help in discovering innovative treatment options for those affected. Xenograft models mimic the natural environment of cancer development, providing a practical approach to investigate breast tumor pathophysiology and develop novel treatment regimens. The MDA-MB-157 epithelial cell line was derived from the pleural effusion of a 44-year-old female patient with metastatic breast cancer in 1972 by Cailleau at M. D. Anderson Hospital and Tumor Institute, as per a 1980 study in Cancer Research. In addition to being positive for the expression of the WNT7B oncogene, the MDA-MB-157 cell line possesses desmosomes, microvilli and tonofilaments at boundaries between cells. This model has helped further breast cancer research such as in the 2014 Breast Cancer Research article (Zhang et al.) which used MDA-MB-157 xenograft mouse models to study mTOR inhibitors, gene expression and protein levels in triple negative breast cancers (TNBC) which are notoriously aggressive and difficult to treat; they verified that the histologic and genomic profiles of the xenografts matched their original patient tumors and that mTOR inhibitors are effective in slowing growth of TNBC but will need combination therapy to be an effective therapeutic regimen. Another Breast Cancer Research study by Tate et al. (2012) used MDA-MB-157 xenografts as a TNBC model to study a histone deacetylase inhibitor (panobinostat, LBH589) and its ability to selectively target TNBC. Their results showed panobinostat treatment decreased proliferation and survival, halted cell cycle progression, initiated partial mesenchymal-to-epithelial transition and increased histone acetylation in vitro and inhibited tumor formation in vivo, suggesting the TNBC therapeutic potential of panobinostat. Finally, Zheng et al. (2015) used MDA-MB-157 to study a technique to circumvent the protective roles of hypoxia and mitophagy against radiotherapy in aggressive tumors. Their results showed an accumulation of TAT-ODD-p53, a fusion p53 protein that inhibited Parkin-mediated mitophagy and increased sensitivity of tumors to radiotherapy in vivo and in vitro.
The MDA-MB-157 cell line is used to create the CDX (Cell Line Derived Xenograft) MDA-MB-157 xenograft mouse model. The MDA-MB-157 xenograft model is triple-negative (lacking estrogen, progesterone and HER2/NEU receptors) and can be targeted by mono- or combination therapies (e.g. paclitaxel, pretubulin inhibitor, panobinostat).
MDA-MB-157 Breast Cancer Xenograft Model: Download ![]()
Download Altogen Labs MDA-MB-157 Xenograft Model PowerPoint Presentation: ![]()
Oncogenes Expression and Characteristics
MDA-MB-157 cells, a mesenchymal-like subtype of triple-negative breast cancer (TNBC), exhibit aggressive migration and invasion properties, largely driven by oncogenic kinase signaling. These cells demonstrate hyperactivation of the PI3K/AKT/mTOR pathway, which promotes survival, proliferation, and metastatic potential. Additionally, mutations in key regulators such as GSK3, WNK1, and p53 contribute to their enhanced motility and resistance to therapy. The dysregulation of MAPK and STAT signaling further amplifies their metastatic behavior by enabling epithelial-to-mesenchymal transition (EMT). Targeting these pathways with phytochemicals like fisetin and quercetin has been shown to suppress migration and metastasis by inhibiting critical kinases and reversing mesenchymal traits. Notably, these oncogenic alterations render MDA-MB-157 cells highly aggressive, making them a valuable model for studying novel anti-metastatic strategies. Understanding their molecular profile is crucial for developing targeted therapies against TNBC.
Genomic Instability and Dysregulated Mitosis in MDA-MB-157 Cells
MDA-MB-157 breast cancer cells exhibit significant karyotypic instability, with chromosome numbers ranging from 52 to 69, reflecting their high degree of genomic aberration. These cells possess a mutated TP53 gene, which impairs DNA damage response and contributes to uncontrolled cell division. Unlike many TNBC subtypes, they retain wild-type BRCA1/2, suggesting alternative mechanisms drive their instability. A key feature of MDA-MB-157 cells is the overexpression of MAD2L2, a regulator of mitotic progression that disrupts proper spindle assembly checkpoint (SAC) function. This leads to frequent mitotic slippage, where cells prematurely exit mitosis without proper chromosome segregation, fueling aneuploidy. Furthermore, defective APC/C (Anaphase Promoting Complex/Cyclosome) regulation results in the accumulation of mitotic substrates, exacerbating chromosomal imbalances. These abnormalities contribute to the aggressive phenotype of MDA-MB-157 cells, making them an important model for studying genomic instability in triple-negative breast cancer (TNBC). Understanding their karyotypic landscape could reveal new therapeutic targets to improve treatment outcomes for TNBC patients.
Tumor Suppression in Triple-Negative Breast Cancer
A study by Shi Y, et al., published by Neoplasia investigates the role of MDA-MB-157 cells in triple-negative breast cancer (TNBC) research, particularly in the context of tumor suppression and therapeutic sensitivity. MDA-MB-157 cells were subcutaneously implanted into xenograft models to evaluate tumor growth and response to treatment. The study emphasizes the involvement of the DAXX protein in modulating tumor progression by repressing RAD51, a key player in homologous recombination-mediated DNA repair. Findings suggest that the overexpression of DAXX leads to a significant reduction in tumor growth, as evidenced by in vivo imaging and histological analysis. Furthermore, suppression of RAD51 sensitized MDA-MB-157 cells to PARP inhibitors, demonstrating a potential therapeutic strategy for BRCA-proficient TNBC cases. The research further explores the combined effect of RAD51 and PARP inhibition in MDA-MB-157-derived tumors. Experimental results indicate that dual inhibition enhances tumor suppression, supporting the concept of synthetic lethality in TNBC treatment. Immunohistochemical staining confirmed increased apoptosis in DAXX-overexpressing tumor tissues. The study underscores the potential for targeting RAD51 in conjunction with PARP inhibitors as an effective approach for treating TNBC, particularly in cases that retain BRCA functionality. Collectively, these findings establish MDA-MB-157 as a critical model for exploring novel therapeutic interventions in TNBC.
A study conducted by Tate CR, et al., published by Breast Cancer Research, investigates the therapeutic potential of panobinostat, a histone deacetylase inhibitor, in targeting triple-negative breast cancer (TNBC) cells, including the MDA-MB-157 cell line. TNBC represents an aggressive breast cancer subtype with limited treatment options due to the absence of hormone receptors and HER2 expression. The study evaluates the effects of panobinostat on TNBC cell proliferation, viability, apoptosis, and tumorigenicity using both in vitro and in vivo models. MDA-MB-157 and other TNBC cell lines were treated with nanomolar concentrations of panobinostat, leading to increased histone acetylation, decreased cell survival, and G2/M phase cell cycle arrest. Notably, panobinostat-induced apoptosis was observed in all tested TNBC cell lines except MDA-MB-468, underscoring cell line-specific responses to treatment.
In vivo xenograft studies further demonstrated panobinostat’s efficacy in reducing tumor growth, highlighting panobinostat’s role in modulating key cancer biomarkers, including the upregulation of E-cadherin (CDH1), suggesting a potential reversal of the epithelial-to-mesenchymal transition (EMT). This finding implies that panobinostat not only inhibits tumor progression but may also reduce metastatic potential. The observed cytotoxic effects, combined with the modulation of tumor suppressor and epithelial marker genes, support the potential of panobinostat as a targeted therapy for aggressive TNBC cases.
Basic study design
- MDA-MB-157 cells are trypsinized and cell viability determined. Trypan blue assay results must exhibit at least 98% cell viability.
- After adjusting cell suspension concentration to one million cells per 100 µL (Matrigel + MDA-MB-157), 10 to 12 week old athymic BALB/C or NOD/SCID mice receive a single subcutaneous injection. All injections are in the rear hind leg flank.
- All injection sites are observed and palpated three times a week until it is determined tumors are established. Tumors are then continually measured (with digital calipers) until an average size of 100-150 mm3 is reached.
- Animal randomization into client determined treatment cohorts and administration of compound of interest is performed.
- Daily tumor measurement and mouse weights (3 times weekly) are recorded. Animals are euthanized at the predetermined size limit.
- Tissue collections are performed and can be snap frozen in LN2 and prepared for histological analysis.
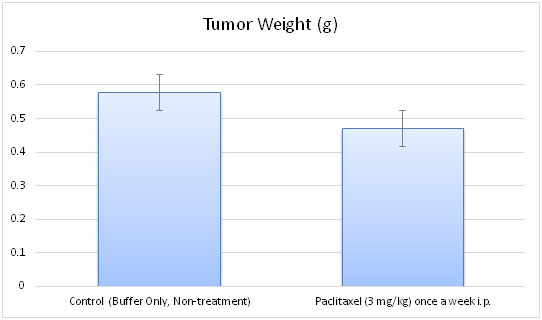
- Tumors are weighed and documented (digital imaging).
Get Instant Quote for
MDA-MB-157 Xenograft Model
Altogen Labs provides an array of laboratory services using over 30 standard Cell Line Derived Xenograft (CDX) models and over 20 PDX models. Researchers investigating the role of specific proteins or gene products in regulating tumor growth can benefit from development of protein overexpression (genetically engineered to ectopically express proteins, tumor suppressors, or oncogenes) and RNAi cell lines with long term gene silencing. Altogen Labs provides quantitative gene expression analysis of mRNA expression (RT-PCR) and protein expression analysis using the WES system (ProteinSimple).
Xenograft animal models are used to assess the effectiveness of drugs against specific types of cancer. New medicines are tested on staged tumor growths that have been engrafted via subcutaneous or orthotopic inoculation in an immunocompromised mouse or rat model. All clinically approved anti-cancer agents have been evaluated with conventional preclinical in vivo models. Xenograft studies can be highly complex, starting with the selection of the appropriate animal model, choice of tumorigenic cell line, administration method, dosing, analysis of tumor growth rates and tumor analysis (histology, mRNA and protein expression levels).
Animal handling and maintenance at the Altogen Labs facility is IACUC-regulated and GLP-compliant. Following acclimatization to the vivarium environment, mice are sorted according to body mass. The animals are examined daily for tumor appearance and clinical signs. We provide detailed experimental procedures, health reports and data (all-inclusive report is provided to the client that includes methods, results, discussion and raw data along with statistical analysis). Additional services available include collection of tissue, histology, isolation of total protein or RNA and analysis of gene expression. Our animal facilities have the flexibility to use specialized food or water systems for inducible gene expression systems.
Following options are available for the MDA-MB-157 xenograft model:
- MDA-MB-157 Tumor Growth Delay (TGD; latency)
- MDA-MB-157 Tumor Growth Inhibition (TGI)
- Dosing frequency and duration of dose administration
- Dosing route (intravenous, intratracheal, continuous infusion, intraperitoneal, intratumoral, oral gavage, topical, intramuscular, subcutaneous, intranasal, using cutting-edge micro-injection techniques and pump-controlled IV injection)
- MDA-MB-157 tumor immunohistochemistry
- Alternative cell engraftment sites (orthotopic transplantation, tail vein injection and left ventricular injection for metastasis studies, injection into the mammary fat pad, intraperitoneal injection)
- Blood chemistry analysis
- Toxicity and survival (optional: performing a broad health observation program)
- Gross necropsies and histopathology
- Positive control group employing cyclophosphamide, at a dosage of 20 mg/kg administered by intramuscular injection to the control group daily for the study duration
- Lipid distribution and metabolic assays
- Imaging studies: Fluorescence-based whole body imaging
