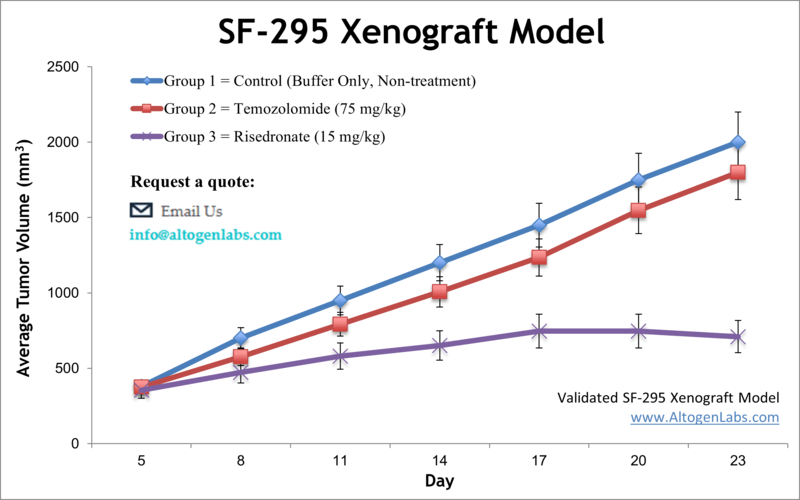
SF-295 xenograft model
SF295 is a human glioblastoma cell line that is commonly used in research to study brain cancer, particularly glioblastoma multiforme (GBM), which is the most aggressive and malignant form of brain cancer. The SF295 cell line was originally derived from a patient with malignant astrocytoma. The American Brain Tumor Association (ABTA) reports that in pediatric malignancies, brain tumors are the most common and are the primary cause of child cancer fatalities. It is estimated that 80,000 adults are diagnosed with brain tumors annually, with more than a third being malignant leading to 17,000 deaths per year. This cell line was established in the late 1980s from a female 67YO patient diagnosed with glioblastoma. SF-295 is identified to be homozygous for PTEN and TP53 and have a late stage phenotype. In vitro cell models (i.e. cancer cell lines) have been essential model systems for exploring the fundamental properties of tumors in preclinical research. Johanns et al. (2016) published a Brain Tumor Pathology study establishing SF-295 as a model for studying telomerase biology in glioblastoma; this is significant as malignant glioma often presents highly recurrent TERT promoter gene mutations. A 1994 study (Plowman et al.) used SF-295 to demonstrate synergism between temozolomide, a methylating agent, and 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU, Carumstine), an alkylating agent, that resulted in equivalent efficacy and lower toxicity compared to individual treatment in brain tumors. In modern research SF-295 is a standard brain tumor model used in a variety of cell line panel projects, including the MD Anderson Cell Lines Project and the NCI-60 cancer cell line panel.
SF295 Brain Cancer Subcutaneous Xenograft Model: Download ![]()
Download Altogen Labs SF-295 Xenograft Model PowerPoint Presentation: ![]()
SF295 Subcutaneous Xenograft Model for Glioblastoma Research
The subcutaneous SF295 xenograft model is widely used in preclinical research to study glioblastoma, a highly aggressive form of brain cancer. Derived from a 67-year-old female patient with glioblastoma, SF295 cells are implanted subcutaneously into immunocompromised mice, typically BALB/C or NOD/SCID strains, to create a tumor microenvironment that mimics the human disease. The model allows for the evaluation of tumor growth dynamics, therapeutic efficacy, and molecular responses to treatment. Tumor volume is monitored regularly, and once tumors reach a predetermined size, they are excised and analyzed for histological and molecular markers. This model is particularly valuable for testing novel drug candidates, assessing drug toxicity, and understanding the genetic alterations characteristic of glioblastoma, such as mutations in PTEN and TP53. By using the SF295 model, researchers can identify promising therapies and better understand glioblastoma progression and resistance mechanisms. It provides a reliable and reproducible platform for developing targeted treatments for this challenging cancer.
Oncogenic Alterations and Drug Resistance in SF295 Glioblastoma Cells
The SF295 cell line, derived from a human glioblastoma, serves as a key model in understanding drug resistance mechanisms in cancer research. Notably, SF295 cells exhibit mutations in key oncogenes, including PTEN and TP53, which contribute to tumor progression and resistance to therapy. The resistance phenotype in SF295 cells is prominently linked to decreased expression of DNA topoisomerase I (Top1), a critical enzyme targeted by many chemotherapy drugs such as camptothecin and its analogs. In studies involving the SF295 sublines SF295/hCPT50 and SF295/BN50, which were selected for resistance to topoisomerase inhibitors, the reduced Top1 expression is primarily regulated at the transcriptional level. This reduction in Top1 impairs the formation of DNA-protein crosslinks, a key process in the action of Top1 inhibitors, thus conferring resistance to these drugs.
In addition to the loss of Top1 expression, the SF295 cell line demonstrates cross-resistance to multiple topoisomerase I inhibitors, but remains sensitive to other classes of drugs, including topoisomerase II inhibitors like mitoxantrone and etoposide. This highlights the significance of Top1 in mediating resistance and suggests the potential use of Top2 inhibitors in treating SF295 models with acquired resistance to Top1-targeting agents. Despite the development of resistance, the SF295 model remains essential for investigating the molecular mechanisms of glioblastoma and testing new therapeutic strategies, particularly in overcoming Top1-related resistance.
Targeting DNA Repair Pathways to Overcome TMZ Resistance in Glioblastoma
In a stduy published by Cancers journal, authors evaluated strategies to overcome temozolomide (TMZ) resistance in glioblastoma (GBM) by enhancing NAD+ bioavailability and inhibiting poly(ADP-ribose) glycohydrolase (PARG). TMZ, a standard treatment for GBM, faces resistance due to high expression of MGMT (O6-methylguanine-DNA methyltransferase) or defects in the mismatch repair (MMR) pathway. The authors hypothesize that increasing NAD+ levels with the precursor dihydronicotinamide riboside (NRH) and inhibiting PARG can potentiate TMZ’s effects. The combination of TMZ, NRH, and PARG inhibitor (PARGi) significantly enhanced cytotoxicity in TMZ-resistant GBM cell lines, including those with MGMT overexpression or MSH6 mutations. This approach led to a hyperaccumulation of poly(ADP-ribose) (PAR), an important signal in the base excision repair (BER) pathway, inhibiting DNA repair. This resulted in elevated DNA damage and apoptosis, highlighting the potential of NRH + PARGi combination therapy in overcoming TMZ resistance. The regimen was effective across multiple GBM cell lines, suggesting its broad applicability for treating resistant GBM.
Metabolic Reprogramming in SF295 Glioma Cells Following Schweinfurthin Treatment
Schweinfurthins, a class of natural compounds, exhibit potent anticancer activity in certain cell lines, with SF295 glioma cells showing remarkable sensitivity at nanomolar concentrations. These compounds induce significant metabolic disruptions in SF295 cells, particularly affecting pathways involved in energy production, such as the tricarboxylic acid (TCA) cycle and oxidative phosphorylation. In addition to metabolic changes, schweinfurthins activate stress-response and signaling pathways, including PDGF and Toll receptor signaling, that are crucial for cellular adaptation to treatment. However, not all cell lines exhibit the same sensitivity; for instance, A549 lung cancer cells are more resistant to schweinfurthins. This resistance is linked to the differential regulation of pathways such as Hedgehog signaling, which is upregulated in A549 cells after treatment. In contrast, SF295 cells do not show such activation, suggesting that the Hedgehog pathway plays a key role in mediating resistance. Interestingly, inhibition of the Hedgehog pathway using specific inhibitors can enhance the sensitivity of resistant cells to schweinfurthin treatment, offering a potential strategy to overcome resistance and improve therapeutic outcomes.
SF295 Brain Cancer Subcutaneous Xenograft Model: Download ![]()
Basic study design
- SF-295 cells are continually grown at conditions of exponential growth. Cells are prepared via trypsinization, with viable cell counts determined using trypan blue exclusion. Cell suspension concentrations are diluted to the working concentration.
- One million SF-295 cells (1 x 10^6 cells) / per 100 uL injection volume in 50% Matrigel solution is inoculated subcutaneously into 10 to 12 weeks old athymic BALB/C (or NOD/SCID) mice. Each animal receives a single subcutaneous (s.c.) injection in the hind leg flank.
- Upon tumor establishment, animals are grouped into cohorts. Injection of the client provided test material is performed according to treatment schedule.
- Whole body mouse weights are recorded up to 3 times a week. Tumor measurements are taken daily. Tumor size limits determine the end of the study. Tumors are excised from the mice, weighed and documented (digital imaging).
- Collected tissues (for downstream analysis) are snap frozen in liquid nitrogen, immersed in RNAlater, or protein/RNA/DNA isolated.
Get Instant Quote for
SF-295 Xenograft Model
SF295 cell line derived from a human glioblastoma. It is commonly used in cancer research as a model for brain tumors. The SF-295 xenograft has been used in studies investigating the efficacy of various treatments for brain tumors, including chemotherapy, radiation therapy, and targeted therapies. Animal handling and maintenance at the Altogen Labs facility are IACUC-regulated and GLP-compliant. Following acclimation to the vivarium environment, mice are sorted according to body mass. The animals are examined daily for tumor appearance and clinical signs. We provide detailed experimental procedures, health reports and data (all-inclusive report is provided to the client that includes methods, results, discussion and raw data along with statistical analysis). Xenograft animal models are used to assess the effectiveness of drugs against specific types of cancer. New medicines are tested on staged tumor growths that have been engrafted via subcutaneous or orthotopic inoculation in an immunocompromised mouse or rat model. All clinically approved anti-cancer agents have been evaluated with conventional preclinical in vivo models. Xenograft studies can be highly complex, starting with the selection of the appropriate animal model, choice of tumorigenic cell line, administration method, dosing, analysis of tumor growth rates and tumor analysis (histology, mRNA and protein expression levels).
Altogen Labs provides an array of laboratory services using over 90 standard Cell Line Derived Xenograft (CDX) models and over 30 PDX models. Altogen Labs provides quantitative gene expression analysis of mRNA expression (RT-PCR) and protein expression analysis using the WES system (ProteinSimple). The dosing of the experimental compound of interest is initiated, for a staged study, when the mean tumor size reaches a specified volume (typically 75-125 mm3).
Following options are available for the SF-295 xenograft model:
- SF-295 Tumor Growth Delay (TGD; latency)
- SF-295 Tumor Growth Inhibition (TGI)
- Dosing frequency and duration of dose administration
- Dosing route (intravenous, oral gavage, subcutaneous)
- SF-295 tumor immunohistochemistry (IHC)
- Blood chemistry analysis
- Gross necropsies and histopathology
- Positive control group employing dox or cyclophosphamide
- Imaging studies: Fluorescence-based whole body imaging
