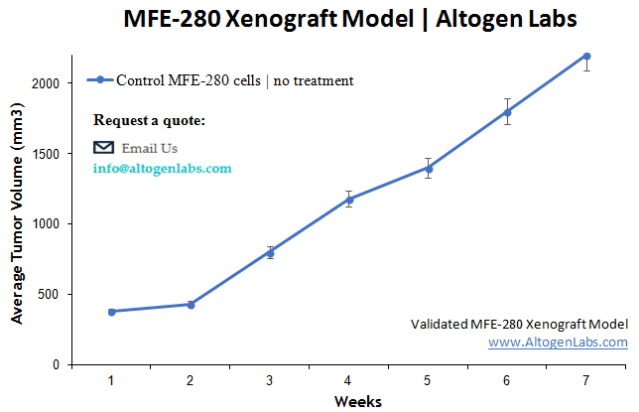
MFE280 Xenofraft Model
Uterine cancer, or womb cancer, can refer to any uterine tissue carcinoma including cervical cancer and endometrial cancer. Risk factors include old age, obesity and HPV infection. Unfortunately there are often no early stage symptoms of uterine cancer, but symptoms may include pelvic pain or irregular vaginal bleeding. The exact causes are still not well understood. The MFE-280 cells are from a 77 yo female patient with endometrial adenocarcinoma. The MFE-280 model has been used in many endometrial cancer research studies. Hall et al. (PLOSONE, 2016) used many cancer lines with mutated FGFR (including MFE-280 cells, which have mutant FGFR S252W) to characterize the novel inhibitor ARQ 087, which is an ATP competitive multi-kinase small molecule inhibitor that has been shown to have anti FGFR activity, against dysregulated FGFR systems. Results indicated that in dysregulated FGFR cell lines, ARQ 087 induced cell cycle arrest and apoptosis as well as in vivo inhibition of xenograft tumor growth. ARQ 087 had already been started in intrahepatic cholangiocarcinoma clinical trials with FGFR2 gene fusions. The MFE-280 cell line is used to create the CDX (Cell Line Derived Xenograft) MFE-280 xenograft mouse model. The MFE-280 xenograft model has been used in endometrial tumor biology studies.
Basic Study Design
- MFE-280 cells are maintained in exponential growth phase under aseptic conditions.
- Cells are trypsinized and cell count viability is determined using a trypan blue exclusion assay (98% of cell viability is required). MFE-280 cell suspension is adjusted to appropriate density.
- Each mouse is singly subcutaneously injected into the right flank with 106 cells in 100-150 µL of a Matrigel-MFE280 cells suspension.
- The injection sites are palpated up to three times weekly until tumors are established to an average size of 100-150 mm3 as measured via digital calipers.
- Animals are randomized into treatment groups. Administration of test compound is performed according to the pre-established treatment schedule.
- Mice weights are measured and recorded 3 times weekly; tumors are measured and recorded daily.
- End of study is reached when tumor size reaches 2,000 mm3 or the predetermined size limit per approved IACUC protocol.
- Final necropsy and tissue collections are performed for appropriate downstream analysis. Tumors are excised, weighed and documented by digital imaging. Tumors and tissues can be stabilized in RNA later reagent, snap frozen in LN2 or prepared for histology.
