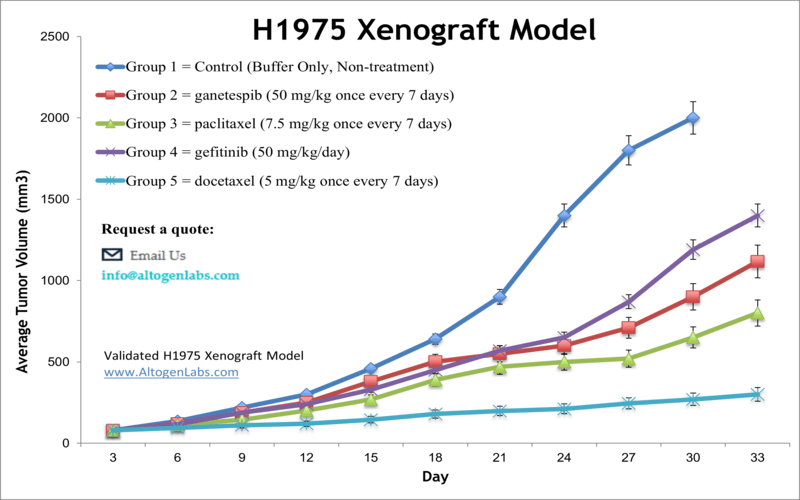
NCI-H1975 xenograft model
The NCI-H1975 epithelial cell line was isolated in 1988 from lung cells taken from a non-smoker female patient with adenocarcinoma. This cell line is a potent tool for performing preclinical tests for lung cancer treatment. Although lung cancer therapeutics have evolved dramatically within last two decades, lung cancer is considered the primary cause of cancer deaths globally. Non-small cell lung cancer (NSCLC) is responsible for the majority of lung cancer cases. New approaches in lung cancer therapy are vital in treating NSCLC patients. A study by Smith et al. published in Targeted Oncology investigated the antitumor efficacy of selective EGFR tyrosine kinase inhibitors (TKIs) and the HSP90 inhibitor ganetespib, alone and in combination, using the NCI-H1975 xenograft model. The results indicate that concurrent administration of ganetespib overcomes erlotinib resistance and significantly improves tumor growth inhibition in erlotinib-resistant NCI-H1975 xenografts. According to the article, combination treatment with both drugs substantially enhances antitumor response and could be a therapy of choice for NSCLC patients. A 2018 Clinical Cancer Research study (Steiner et al.) used the H1975 xenograft model to study the effects of mutated epidermal growth factor receptor (EGFR) with treatment with cetuximab, a monoclonal IgG1 antibody also known as Erbitux. EGFR is mutated (somatic) in 10% of patients which affects EGFR targeted therapy; results demonstrated that cetuximab had antitumor activity with both wild type and mutated EGFR and that combination treatments with cisplatin or docetaxel increased these effects which is particularly relevant for chemorefractory NSCLC. In 2015 Cross et al. released a study in Cancer Discovery using the NCI-H1975 model to look at overcoming T790-mediated resistance to EGF receptor tyrosine kinase inhibitors (EGFR TKIs). They found that treatment with AZD9291, an irreversible dual inhibitor of EGFRm+ and T790M inhibits tumor cell growth and signaling pathways in advanced NSCLC. The H1975 cell line (human lung) is used to create the CDX (Cell Line Derived Xenograft) NCI-H1975 xenograft mouse model. The H1975 xenograft model is a mutated EGFR (T790M, L858R) expressing model used for preclinical studies of monotherapies or in combination (e.g. cetuximab, cisplatin, gemcitabine, docetaxel).
NCI-H1975 Lung Cancer Xenograft Model: Download ![]()
Download Altogen Labs NCI-H1975 Xenograft Model PowerPoint Presentation: ![]()
Get Instant Quote for
NCI-H1975 Xenograft Model
Subcutaneous H1975 Lung Cancer Xenograft Model
The subcutaneous H1975 lung cancer xenograft model involves the implantation of H1975 cells under the skin of immunocompromised mice, allowing researchers an opportunity to study of tumor growth and therapeutic responses of H1975 in vivo. This model also effectively replicates key aspects of non-small cell lung cancer (NSCLC) harboring EGFR mutations, providing a reliable platform for evaluating the efficacy of targeted therapies and combination treatments. Its consistent tumor formation and predictable growth kinetics make it an essential tool for preclinical assessment of novel anticancer agents. In preclinical studies at Altogen Labs, H1975 cells are cultured to the exponential growth phase, prepared using trypsin-EDTA, and suspended at 1 × 10⁶ cells in 150 µL of a 50% Matrigel solution for subcutaneous injection into the flank of athymic BALB/c immunocompromised mice. Tumor growth is monitored until reaching 100–150 mm³, after which mice are randomized into treatment groups and administered the test compound according to a set schedule, with regular monitoring of body weight and tumor size. The study concludes when tumors reach 2,000 mm³, followed by necropsy, tumor excision, weighing, imaging, and sample preservation for histological or molecular analysis.
H1975 Lung Cancer Cells Oncogenic Pathways and Targeted Therapy in EGFR-Mutant NSCLC
The H1975 cell line, derived from lung adenocarcinoma, is a key model due to its dual EGFR mutations: the sensitizing L858R mutation and the resistance-associated T790M mutation. This combination confers intrinsic resistance to first-generation TKIs like Gefitinib and Erlotinib. H1975 cells harbor a p53 mutation, further contributing to their complex oncogenic profile and influencing therapeutic resistance. Additionally, H1975 is possibly useful in evaluating third-generation EGFR-TKIs such as Osimertinib, designed to overcome T790M-mediated resistance. However, even Osimertinib resistance emerges, prompting investigations into combination therapies and alternative pathways, including targeting IGF1R and p53 pathways.
The H1975 xenograft model offers a comprehensive platform for evaluating tumor progression, therapeutic efficacy, and biological responses. At Altogen Labs, key study options include Tumor Growth Delay (TGD) and Tumor Growth Inhibition (TGI) assessments, along with flexible dosing strategies in terms of frequency, duration, and various administration routes (e.g., intravenous, oral gavage, intratumoral). Advanced analyses are available, such as immunohistochemistry, blood chemistry, lipid metabolism assays, and fluorescence-based imaging for in vivo tumor visualization. Researchers can choose alternative engraftment sites for metastasis studies and incorporate toxicity, survival evaluations, and detailed necropsy with histopathology. A positive control group using cyclophosphamide (50 mg/kg intramuscularly) can be included to benchmark therapeutic responses.
Epigenetic Therapy in H1975 Xenograft Models for Lung Cancer
In the study by Yang et al., published in OncoTargets and Therapy journal, the H1975 lung cancer cell line was used alongside xenograft models to investigate the therapeutic potential of combining the DNA methyltransferase inhibitor azacitidine (5-AZA) and the histone deacetylase inhibitor trichostatin A (TSA). The combined treatment significantly inhibited H1975 cell proliferation and viability in vitro and suppressed tumor growth in vivo. This effect was associated with the disruption of the AKT signaling pathway and the upregulation of tumor-suppressor genes like TFF1 and VCAM1. Notably, in the H1975 xenograft model, the co-treatment delayed tumor formation and reduced tumorigenic potential.
Basic study design
- Prior to trypsinization of H1975 cells, they are continually grown at a phase of exponential growth. Viable cell counts are determined using trypan blue and concentration is adjusted to appropriate density needed for xenotransplantation.
- 11-12 week old athymic BALB/C mice receive subcutaneous injection into the hind leg. Each animal receives a 160 µL injection containing one million cells of the Matrigel + H1975 cell suspension.
- Tumors are calipered until growth reaches average sizes of 75-150 mm3. At this point, grouping of mice into treatment cohorts and injections of the compound of interest is started according to treatment schedule.
- Mouse body weights are documented up to 2-3 times weekly, with tumors calipered daily.
- When the end of study parameters are reached (i.e. maximum tumor size), animals are euthanized. All tissues collected snap frozen, submerged in a stabilizing solution (e.g. RNA Later reagent) or nucleic acids isolated. Tumors are excised from the mice and weighed.
All clinically approved anti-cancer agents have been evaluated with conventional preclinical in vivo models. Xenograft studies can be highly complex, starting with the selection of the appropriate animal model, choice of tumorigenic cell line, administration method, dosing, analysis of tumor growth rates and tumor analysis (histology, mRNA and protein expression levels).Xenograft animal models are used to assess the effectiveness of drugs against specific types of cancer. New medicines are tested on staged tumor growths that have been engrafted via subcutaneous or orthotopic inoculation in an immunocompromised mouse or rat model. Altogen Labs provides an array of laboratory services using over 120 validated CDX and PDX models. Researchers investigating the role of specific proteins or gene products in regulating tumor growth can benefit from development of protein overexpression (genetically engineered to ectopically express proteins, tumor suppressors, or oncogenes) and RNAi cell lines with long term gene silencing. Altogen Labs provides quantitative gene expression analysis of mRNA expression (qPCR) and protein expression analysis using the WES system. We provide detailed experimental procedures, health reports and data (all-inclusive report is provided to the client that includes methods, results, discussion and raw data along with statistical analysis). Additional services available include collection of tissue, histology, isolation of total protein or RNA and analysis of gene expression.
Following options are available for the H1975 xenograft model:
- H1975 Tumor Growth Delay (TGD; latency)
- H1975 Tumor Growth Inhibition (TGI)
- Dosing frequency and duration of dose administration
- Dosing route (intravenous, intratracheal, continuous infusion, intraperitoneal, intratumoral, oral gavage, topical, intramuscular, subcutaneous, intranasal, using cutting-edge micro-injection techniques and pump-controlled IV injection)
- H1975 tumor immunohistochemistry
- Blood chemistry analysis
- Toxicity and survival (optional: performing a broad health observation program)
- Gross necropsies and histopathology
- Positive control group employing Dox or Cyclophosphamide, at a dosage of 10-50 mg/kg
- Lipid distribution and metabolic assays
