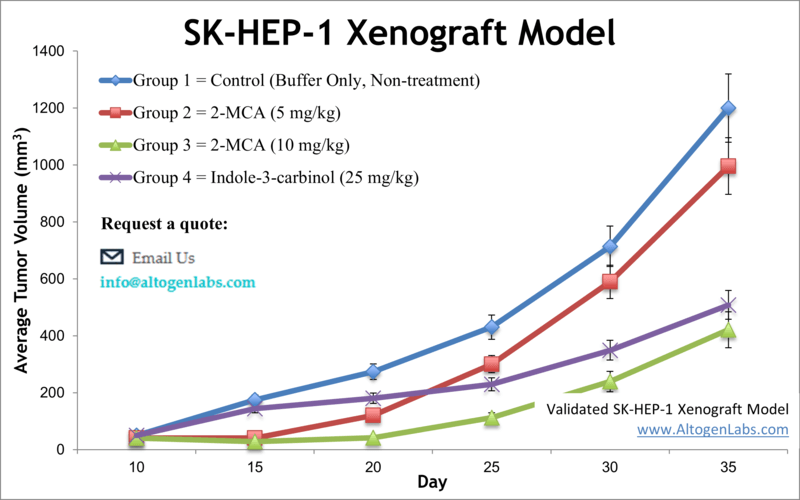
SK-HEP-1 xenograft model (subcutaneous and metastatic)
SKHEP1 is a human hepatocellular carcinoma (HCC) cell line that is commonly used in research to study liver cancer. The SKHEP1 cell line was originally derived from a patient with liver adenocarcinoma. Liver cancer is a devastating disease that is characterized by a high risk of recurrence and limited treatment options. The average survival after diagnosis is roughly from 6 to 20 months, depending on the stage at diagnosis. Preclinical animal models are potent tools in liver cancer research for selecting drug candidates for clinical development. The SK-HEP-1 epithelial cell line was isolated from the ascitic fluid of a 52-year-old Caucasian male patient with adenocarcinoma of the liver in 1971 by G. Trempe and J. Fogh. Tai et al. released a 2018 study outlining the use of SK-HEP-1 as liver sinusoidal endothelial cells rather than hepatocellular carcinoma cells as a reflection of their gene expression, protein levels and morphological phenotype (tubular formations, fenestrae with no diaphragms) which is important to consider when selecting a cell model for your research. This is also reflected in the study by Eun et al. (2014) demonstrating the SK-HEP-1 model to represent a unique mesenchymal to stem cell transformation, as the cells do not exhibit expression of liver specific transcripts, with metastatic potential. Prior to this a 2014 article published in Oncogene, demonstrates that the SK-HEP-1 xenograft model is suitable for examining tumor growth and metastasis simultaneously. The study indicates that Zcchc11, a repressor of let-7 maturation, promotes tumor growth and metastasis in vivo and is overexpressed in human cancers, which has implications to develop new strategies for cancer therapy. A 2015 Molecular Oncology article (Hou et al.) used the SK-HEP-1 xenograft model to demonstrate that the plasma membrane protein gp96 is associated with poor prognosis in hepatic cell carcinoma (HCC) because it regulates a uPAR-mediated increase in growth, survival and invasion (and decrease in KDELR1, an ER receptor). Data also demonstrated that siRNA targeting of the mgp96 and uPAR interaction decreases tumor growth and metastasis, supporting this interaction as a target for cancer therapy and eventual disease free survival (DFS). Clinical Cancer Research released a 2014 article by Xiang et al. using the SK-HEP-1 xenograft model to characterize the mechanism of anticancer action of the MET and VEGFR-2 inhibitor cabozantinib as a potential way to overcome resistance to adjuvant treatment with sorafenib. Cabozantinib treatment demonstrated a decrease in angiogenesis, proliferation and an increase in apoptosis. The SK-HEP-1 cell line (human liver) is used to create the CDX (Cell Line Derived Xenograft) SK-HEP-1 xenograft mouse model. The SK-HEP-1 xenograft model is utilized in monotherapy studies or in combination antitumor activity studies of sphingosine kinase 2 inhibitors (e.g. ABC-294640) in combination with sorafenib (mechanism of action thru suppression of MAPK pathway; decreased levels of phosphorylated ERK).
Download Altogen Labs SKHEP1 Xenograft Model PowerPoint Presentation: ![]()
Basic study design
- SK-HEP-1 cells are trypsinized while under exponential phase growth. Trypan blue is used to determine viable cell counts. The total cell suspension concentration is adjusted to the necessary density for injection.
- Athymic BALB/C or NOD/SCID mice (10-12 w.o.) receive a single, subcutaneous injection (s.c.) in one hind leg containing 1 x 106 cells (vol = 100-200 µL of Matrigel + SK-HEP-1 cells suspension).
- Palpation of the injection sites are performed three times weekly to determined tumor establishment. Tumors are calipered to monitor progression of tumor growth until a size of 50-150 mm3 is reached.
- Animals are sorted into cohorts and the test material is administered.
- Tumors are monitored (daily measurements) along with mouse body weights logged.
- The in-life portion of the study is reached when tumor size reaches 2,000 mm3 (or the study size limit per approved IACUC protocol). Necropsies are performed and tissues are collected. Tumors are weighed and digitally imaged. As directed by the client, collected tissues can be frozen, prepared for histology or nucleic acids isolated.
Metastatic Model
CDX models are mouse xenografts used in pre-clinical therapeutic studies. However, as primary tumors proliferate they invade surrounding tissue, become circulatory, survive in circulation, implant in foreign parenchyma and proliferate in the distant tissue. This result leads to an extremely high percentage of death in cancer patients due to metastasis. Metastatic tumor mouse models are utilized to develop novel therapeutic agents that target metastasis (anti-metastatic therapeutics).
To create a metastatic model, the cell line of interest is transfected with vectors containing green fluorescent protein (GFP) or luciferase. Maintained under antibiotic selection, only cells containing the integrated vector will survive. The new cell line clones are capable of stably expressing the gene of interest and are used in metastatic mouse model studies. Although each new cell line clone may contain its own inherent difficulties, the new cell line contains the ability to track internal tumor progression via bioluminescence (luciferase fluorescence after injecting luciferin) or fluorescence (GFP). Internal orthotopic and metastatic tumor growth (not palpable) can now be measured throughout the study, enabling a researcher to gain more insight and additional data in contrast to relying on end of study tumor weight measurements.
Case Study: U87-luc Xenograft Model
An example of Altogen Labs utilizing a luciferase expressing cell line to monitor orthotopic tumor growth is exhibited below. The same ideology of tumor observation is incorporated in metastatic tumor models.
Luciferase expressing U87-luc cells were implanted and tumors allowed to grow. Tumor growth was monitored in a Night Owl (Berthold Technologies) imaging system 10 minutes after an intraperitoneal (IP) injection of the luciferin substrate. As seen in the example below, luciferase expression (measured as photons emitted) in the U87-luc model grants the researcher a visual image and quantifiable metric for orthotopic or metastatic tumor progression.
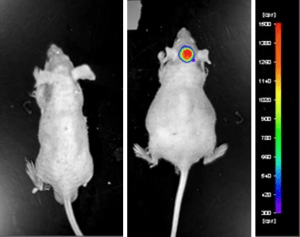
Figure 1. Luciferase expression in U87-luc orthotopic model. Control and implanted glioma mouse model fluorescence was analyzed 10 minutes after intraperitoneal luciferin injection.
View full details of the case study by clicking here.
Get Instant Quote for
SK-HEP-1 Xenograft Model
Xenograft animal models are used to assess the effectiveness of drugs against specific types of cancer. New medicines are tested on staged tumor growths that have been engrafted via subcutaneous or orthotopic inoculation in an immunocompromised mouse or rat model. All clinically approved anti-cancer agents have been evaluated with conventional preclinical in vivo models. Xenograft studies can be highly complex, starting with the selection of the appropriate animal model, choice of tumorigenic cell line, administration method, dosing, analysis of tumor growth rates and tumor analysis (histology, mRNA and protein expression levels). We provide detailed experimental procedures, health reports and data (all-inclusive report is provided to the client that includes methods, results, discussion and raw data along with statistical analysis). Additional services available include collection of tissue, histology, isolation of total protein or RNA and analysis of gene expression. Our animal facilities have the flexibility to use specialized food or water systems for inducible gene expression systems.
Following options are available for the SKHEP1 xenograft model:
- SK-HEP-1 Tumor Growth Delay (TGD; latency)
- SK-HEP-1 Tumor Growth Inhibition (TGI)
- Dosing frequency and duration of dose administration
- Dosing route (intravenous, intratracheal, continuous infusion, intraperitoneal, intratumoral, oral gavage, topical, intramuscular, subcutaneous, intranasal, using cutting-edge micro-injection techniques and pump-controlled IV injection)
- SK-HEP-1 tumor immunohistochemistry
- Alternative cell engraftment sites (orthotopic transplantation, tail vein injection and left ventricular injection for metastasis studies, injection into the mammary fat pad, intraperitoneal injection)
- Blood chemistry analysis
- Toxicity and survival (optional: performing a broad health observation program)
- Gross necropsies and histopathology
- Positive control group employing cisplatin, at a dosage of 15-25 mg/kg
