Altogen Labs provides in vivo imaging services for drug development and biodistribution studies: U87 orthotopic glioma xenograft animal model study ![]()
by Altogen Labs, 11200 Manchaca Rd, Suite 203 Austin TX 78748 USA
Introduction
In vivo imaging has emerged as a critical tool in visualization in preclinical research studies related to the development of new therapeutic agents [1]. The term “in vivo” refers to the performance of these preclinical research studies with live animals, and most commonly with small rodents such as mice or rats. Never before has the path from our knowledge of molecular changes to clinical decisions been as clear as with the techniques such as in vivo imaging application. In vivo imaging studies can actually range from the molecular level to cells, tissues and organs and can be useful in longitudinal studies in the context of observing responses to physiological changes [2].
In vivo imaging technology systems can be grouped into systems focused on molecular structures and morphological/anatomical structures. In vivo imaging technology focused on anatomical structures includes magnetic resonance imaging (MRI), computer tomography or CT, high-frequency ultrasound while imaging technology focused on molecular structures includes positron emission tomography or PET, fluorescence, bioluminescence and single photon emission computed tomography or SPECT.
MRI or magnetic resonance imaging is a non-invasive technique that utilizes the nuclear magnetic alignment of the atoms in a magnetic field as the basis for imaging [3]. MRI machines work by generating powerful magnetic fields via radiofrequency coils and an analysis of the alignment of paramagnetic atoms (hydrogen, manganese, and gadolinium). The imaging itself is a product of the relaxation of the atoms that have been exposed to the powerful magnetic fields and are returning to their normal alignment. Different tissue types will have different resonance characteristics and this difference will create an image. MRI technology produces images with a good contrast resolution and is excellent for distinguishing between different types of tissue. MRI is a great option in the world of in vivo imaging because it is safer than imaging technologies that utilize radiation.
Computer tomography (CT) technology works via radiation focused around the test subject that is placed in the scanner [4]. X-rays are emitted and the x-rays attenuate differently depending on the type and density of the tissue that is passing through. The image is a result of sensors picking up these x-rays. As the scanner is able to rotate, a three dimensional image can be produced based upon the 2-D imaged taken by the rotating CT scanner.
PET or positron emission tomography is a type of in vivo imaging technology that works by recording gamma rays emitted from the subject [5]. The radiation in the form of the high-energy gamma rays is a result of biological molecules that are positron emitting such as 18F-FDG or fludeoxyglucose that are injected into the mammalian test subject. Sensors at either end of a PET machine receive the gamma rays that are close to 180 degrees apart as the radioisotopes decay. These emission events are localized and produce date that can be transformed into images.
Bioluminescence imaging technology relies on enzyme reactions that produce light that is then captured by cameras that are highly light sensitive (Charged Coupled Device Cameras) [6]. Fluorescence imaging technology utilizes the excitement of fluorochromes in vivo with an external source. Traditionally, GFP and RFP are the fluorochromes that have been used, however, IR fluorescent proteins and near-IR dyes are also utilized because there is less of an autofluoresence effect of tissue at these wavelengths [7].
SPECT, or single photon emission computed tomography is similar to PET, but unlike PET, SPECT utilizes radioisotopes that emit gamma rays directly, rather than from the annihilation event of an electron and a positron [8]. A gamma ray camera rotates around the test subject and captures the direct rays to create in vivo images.
The different types of in vivo imaging techniques have their respective advantages and disadvantages and therefore selecting the right imaging strategy is dependent upon the unique preclinical research study.
Research Study: In vivo imaging of U87-Luc orthotopic glioma xenograft animal model
Materials and Methods
This study was designed to understand the multiple dose intravenous efficacy of test compound in nude mice with orthotopic model of glioma. The objective of this study was to evaluate the efficacy of test article upon intravenous administration with multiple doses on the reduction of glioma tumor volume in nude mice implanted with U87 cells expressing luciferase.
U87 cells were obtained from ATCC U87 cell line and cultured in 5% CO2 humidified atmosphere at 37°C using in DMEM medium (ATCC) supplemented with 10% FBS (ATCC).
U87 cells were stably transfected with plasmid DNA encoding luciferase gene. Antibiotic selection was completed in 8 weeks. Single colony isolation was completed by serial dilution, followed by drug selection of cell colonies. Stable expression of luciferase gene was confirmed on weeks 4, 6, and 8 using E2940 Dual-Glo Luciferase Assay System.
Intravenous (IV) administrations of compounds were done using Genie Touch Syringe Pump (Kent Scientific). A metered syringe pump was programmed to deliver tail vein IV injection volume of 200 ul. Terumo Surshield safety winged infusion sets were used for tail vein administration.
RESULTS
Night Owl in vivo imaging instrument (Berthold Technologies) with the associated IndiGO software was used to perform the in vivo imaging study. Luciferase expression was analyzed after intraperitoneal luciferin injection as described in Berthold Technologies application note “Luciferin bioavailability in mice during in-vivo imaging by Arnaud Pillon et al”.
Briefly, 125 mg/kg (bodyweight) of D-Luciferin was administered i.p. 10 minutes prior to making luciferase measurements. Animals received i.p. anesthesia. Two types of signal were recorded: photo and luminescence (Fig.1). Experimental conditions for photo data acquisition: Sample Size = 299.71, Sample Height = 15 mm, Camera Gain = Low, EM Gain = 0, Camera Readout = Fast, Exposure Time = 0.1 s, Illumination = 10%, x-Binning = 1, y-Binning = 1. Experimental conditions for luminescence data acquisition: Sample Size = 299.71, Sample Height = 15 mm, Camera Gain = Low, EM Gain = off, Camera Readout = Slow, Exposure Time = 120 s, Illumination = 0%, x-Binning = 2, y-Binning = 2.

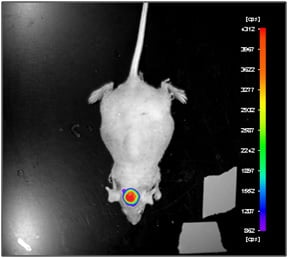
Figure 1. Luciferase expression was analyzed after intraperitoneal luciferin injection in in nude mice with orthotopic model of glioma.
Conclusion
Glioma cell line U87-Luc stably expressing luciferase and orthotopic U87-Luc xenograft model were developed. Experimental protocol to image orthotopic model of glioma was performed. A glioma model was visualised effectively using the Night Owl in vivo imaging after D-Luciferin administration. In vivo imaging as well as these types of multimodality technologies has vast applications across preclinical fields including neurology, cardiology, oncology and infectious diseases.
References
- De Jong, M., J. Essers, and W.M. van Weerden, Imaging preclinical tumour models: improving translational power. Nature reviews. Cancer, 2014. 14(7): p. 481-93.
- O’Farrell, A.C., et al., Non-invasive molecular imaging for preclinical cancer therapeutic development. British journal of pharmacology, 2013. 169(4): p. 719-35.
- Chatham, J.C. and S.J. Blackband, Nuclear magnetic resonance spectroscopy and imaging in animal research. ILAR J, 2001. 42(3): p. 189-208.
- Dawood, A., S. Patel, and J. Brown, Cone beam CT in dental practice. Br Dent J, 2009. 207(1): p. 23-8.
- Cherry, S.R. and S.S. Gambhir, Use of positron emission tomography in animal research. ILAR J, 2001. 42(3): p. 219-32.
- Close, D.M., et al., In vivo bioluminescent imaging (BLI): noninvasive visualization and interrogation of biological processes in living animals. Sensors (Basel), 2011. 11(1): p. 180-206.
- Kelly, K.A., et al., Novel peptide sequence (“IQ-tag”) with high affinity for NIR fluorochromes allows protein and cell specific labeling for in vivo imaging. PloS one, 2007. 2(7): p. e665.
- Rahmim, A. and H. Zaidi, PET versus SPECT: strengths, limitations and challenges. Nucl Med Commun, 2008. 29(3): p. 193-207.
