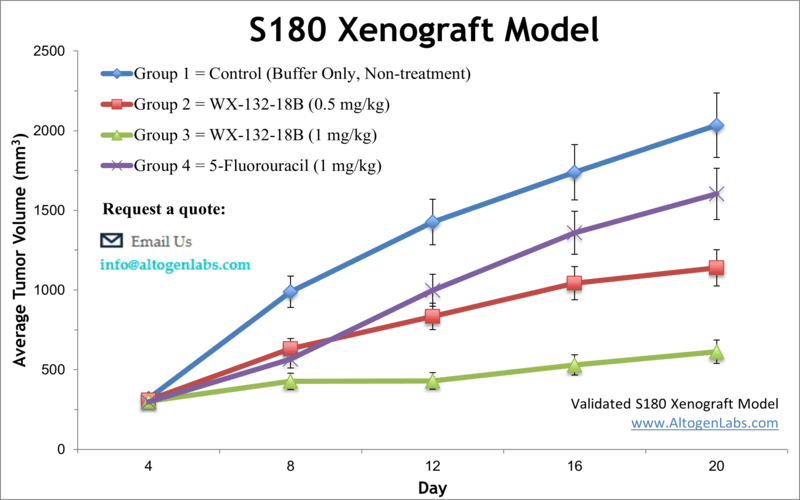
S180 allograft model
S180 cells refer to a cell line that was derived from a mouse sarcoma tumor. The S180 cell line is known to be highly aggressive and invasive, making it a useful model for studying the mechanisms of cancer metastasis. It has been used to investigate the molecular mechanisms underlying cancer cell proliferation, differentiation, and apoptosis, as well as to test potential anti-cancer drugs. Sarcomas are cancers of bones and connective tissues that comprise roughly 20 percent of all childhood malignancies, but are quite rare in adults, according to the Sarcoma Foundation of America. Sarcomas are divided into two main subtypes, bone sarcomas and soft tissue sarcomas. The S180 cell line was initially isolated from a soft tissue tumor in a Swiss mouse. A 2017 study by Guan et al. published in Oncotarget investigated the antitumor activity of a novel microtubule-inhibiting agent (MIA) WX-132-18B using the S180 allograft model. The article indicates that WX-132-18B shows the same cellular phenotypic profile as the classic MIAs, such as colchicine, vincristine and taxol, and triggers tumor cell apoptosis. These findings report that WX-132-18B results in an inhibition on tumor volume and tumor weight in the S180 allograft model and has potent antitumor activity in vivo. A 2012 study by Wang et al. used the S180 allograft model to study the antitumor effects of fucoxanthin, a natural marine carotenoid isolated from sargassum. Results demonstrated that fucoxanthin treatment inhibited tumor growth and promoted apoptosis by altering bcl-2 and caspase-3 levels. Treatment also resulted in a decrease of epidermal growth factor receptor (EGFR) levels as well as signal transducers and activators of transcription 3 (STAT3) proteins, which provides a mechanism by which fucoxanthin targets tumors. Wang et al. published an article (2008) using the S180 allograft model to investigate the antitumor effects of raddeanin A, a triterpenoid saponin isolated from Anemone raddeana Regel. IC50 and LD50 of raddeanin A treatment were measured and was overall deemed to be a potential anticancer agent due to dose dependent growth inhibition. Finally, a 2010 study (Wu et al.) tested the antitumor effects of ATWLPPR-NLLMAAS, a novel chimeric di-heptapeptide that binds to NRP-1 and inhibits VEGF and Ang-1/2 binding to Tie-2. Treatment of S180 allografts with this peptide resulted in decreased angiogenesis as evidenced by microvessel density, inhibition of tumor growth and limited toxicity. These results support the consideration of this chimeric peptide as a potential chemotherapy. The S180 cell line (mouse sarcoma) is used to create the S180 allograft mouse model. The S180 mouse model has been used to test the antitumor activity of saponins (e.g. Raddeanin A) and mitochondrial complex inhibitors (e.g. annonaceous acetogenins ACGs).
S180 Allograft Tumor Model: Download ![]()
Download Altogen Labs S180 Allograft Model PowerPoint Presentation: ![]()
Exploring Tumor Spread with Metastatic S180 Models
Metastatic S180 models are crucial for studying the spread of sarcoma cells to distant organs, particularly the lungs, and for evaluating therapeutic approaches targeting metastasis. In these models, S180 cells are injected into immunocompromised mice, often intravenously, to mimic the process of cancer dissemination. The S180 tumor model is frequently used to study lung metastasis, as the cells have a natural tendency to spread to this organ. This model provides insight into key aspects of metastasis, such as the initial seeding of cancer cells, the formation of metastatic lesions, and the tumor microenvironment’s role in supporting secondary growth. Researchers use metastatic S180 models to test the efficacy of drugs aimed at inhibiting metastasis or promoting tumor regression. These models are particularly useful for studying the molecular mechanisms underlying metastasis and evaluating treatments that target metastatic progression.
Oncogenes and Host Susceptibility in S180 Tumor Metastasis
The S180 sarcoma cell line is a widely used experimental model for studying tumor metastasis and oncogene-driven cancer progression. These cells exhibit metastatic potential due to genetic alterations that enable them to evade immune recognition, particularly through beta-2 microglobulin deficiency and MHC class I destabilization. As a result, S180 cells can proliferate unchecked in various inbred mouse strains, forming aggressive tumors in the lungs when introduced via the bloodstream. Recent studies highlight strain-specific differences in susceptibility to pulmonary metastasis, with some genetic backgrounds exhibiting complete tumor clearance, suggesting an interplay between oncogenic drivers and host immune responses. Additionally, oncogene-driven factors regulating adhesion, extravasation, and proliferation are key determinants of metastatic efficiency in S180 tumors. Immune-mediated mechanisms, particularly T-cell activity, have been shown to play a crucial role in tumor clearance, as T-cell depletion significantly enhances S180 metastasis. Understanding the genetic and immune interactions that dictate S180 tumor behavior provides valuable insight into metastasis regulation and potential therapeutic interventions.
S180 Sarcoma Suppression through DVDMS
In a study by Xiong W, et al. published by Scientific Reports journal, explores the anti-tumor potential of a novel sonosensitizer, sinoporphyrin sodium (DVDMS), in combination with multiple focused ultrasound treatments against S180 sarcoma cells. The research highlights the efficacy of this sonodynamic therapy (SDT) in both in vitro and in vivo models, demonstrating significant suppression of tumor growth, increased apoptosis, and inhibition of angiogenesis. The S180 sarcoma model was used to evaluate the therapeutic effects, revealing that DVDMS accumulates preferentially in tumor cells, enhancing its cytotoxic effects upon ultrasound activation. Notably, the combination therapy resulted in an 89.82% tumor weight inhibition ratio, far surpassing the efficacy of ultrasound alone (32.56%) or a single treatment of DVDMS-SDT (59.33%). Mechanistically, the therapy promoted apoptosis via mitochondrial pathways, inhibited cell proliferation by reducing PCNA expression, and suppressed angiogenesis by downregulating VEGF levels. DVDMS-SDT did not cause significant toxicity or weight loss in treated animals, suggesting its potential as a safe and effective cancer therapy. These findings position S180 sarcoma as a valuable model for investigating SDT and provide strong evidence that DVDMS-mediated SDT could serve as a promising non-invasive treatment strategy for solid tumors.
Buckwheat Polysaccharide as an Adjuvant Therapy for S180 Sarcoma
Another study conducted by Fan DJ, et al., published by Archives of Medical Science journal, investigates the effects of buckwheat polysaccharide (BP) as an adjuvant therapy for S180 sarcoma, a widely used experimental tumor model. S180 tumor-bearing mice were treated with varying doses of BP, either alone or in combination with cyclophosphamide (CTX), to evaluate tumor suppression, immune function, and survival outcomes. While BP did not significantly inhibit tumor growth, it markedly improved lifespan and survival rates, with the medium-dose group showing the highest benefits. Additionally, BP enhanced immune function by increasing thymus and spleen indexes, which were otherwise suppressed by CTX treatment. Flow cytometry analysis revealed that BP arrested S180 tumor cells in the G0/G1 phase, indicating potential anti-proliferative effects. The combination of BP and CTX provided further protective effects by mitigating CTX-induced toxicity without compromising its tumor-suppressive efficacy. Although BP did not directly eliminate S180 sarcoma, its immune-enhancing properties and ability to improve survival suggest its potential as a complementary cancer therapy.
Basic study design
- S180 cells grown under exponential growth are collected and tested for viability (98-99% cell viability required). Cell suspension counts are adjusted to concentrations such that a 120-140 µL injection of the suspension (Matrigel + S180 cells) contains 1 x 106 total cells.
- Each mouse receives a single, subcutaneous (s.c.) injection. The hind leg of each BALB/C mouse (10 to 12 weeks) receives the cell inoculation.
- The injected sites are palpated and monitored pending tumor establishment. Tumors are calipered until an expected size of 75-100 mm3 is reached to begin the in-life portion of the study.
- Animals are sorted into treatment groups exhibiting equal tumor deviation dispersal. Compound administration is performed following the treatment schedule.
- Mouse body weights are recorded (bi- or tri-weekly) and tumors are measured (daily). The end of study is reached when tumor sizes reach 2,000 mm3. Necropsy and tissue collections are defined in the quote.
- Excised tumors are weighed and digitally imaged. Standard gross necropsies are performed to collect for downstream analysis. Per client request, tumors/tissues can be snap frozen or immersed in RNAlater.
Get Instant Quote for
S180 Allograft Model
Altogen Labs facility is IACUC regulated and GLP compliant. Following acclimation to the vivarium environment, mice are sorted according to body mass. The animals are examined daily for tumor appearance and clinical signs. We provide detailed experimental procedures, health reports and data (all-inclusive report is provided to the client that includes methods, results, discussion and raw data along with statistical analysis).
Following options are available for the S180 allograft model:
- S180 Tumor Growth Delay (TGD; latency)
- S180 Tumor Growth Inhibition (TGI)
- Dosing frequency and duration of dose administration
- Dosing route (intravenous, intratracheal, continuous infusion, intraperitoneal, intratumoral, oral gavage, topical, intramuscular, subcutaneous, intranasal, using cutting-edge micro-injection techniques and pump-controlled IV injection)
- S180 tumor immunohistochemistry
- Alternative cell engraftment sites
- Blood chemistry analysis
- Toxicity and survival (optional: performing a broad health observation program)
- Gross necropsies and histopathology
- Positive control group employing cyclophosphamide, at a dosage of 20-25 mg/kg administered by intramuscular injection to the control group daily for the study duration
