PC3 xenograft model (subcutaneous and metastatic)
Prostate cancer is one of the most frequent malignancies among older males. It remains a primary cause of cancer death in men in many developed countries. The American Urological Association recommends prostate cancer screening for all men ages 55 to 69 years. The PC3 cell line was isolated from the bone marrow metastasis of a 62-year-old Caucasian male with prostate cancer after androgen suppression therapy. PC3 is the classical and well-studied cell line extensively employed for human prostate cancer research. PC-3 cells exhibit high metastatic potential. A 2016 study by Li et al. published in Biological Research investigated the role of recombinant trichosanthin (rTCS) on prostate cancer cells PC3 in vitro and in vivo using the PC3 xenograft model. rTCS inhibited proliferation of PC3 cells in a dose-dependent manner and decreased tumor volume weight significantly. IL-2 enhanceed the inhibitory effect of trichosanthin on PC3 cell, overall suggesting that the combination of IL-2 and rTCS could be used as a potential therapeutic strategy for prostate cancer patients. Rij et al., in the Journal of Nuclear Medicine (2018), used the PC3 xenograft model to characterize the efficacy of hRS7, a humanized iGG1 monoclonal antibody against EGP-1/TROP2, for treatment of prostate cancer. The group used a radiolabeled antibody for radioimmunoimaging techniques including PET and SPECT in order to examine histology and concluded that this technique was able to successfully selectively target prostate tumor cells. A 2017 Nature study conducted by Gui et al. also used the PC3 model, but to characterize the anticancer effects of Litchi, or Chinese Cherry, on prostate cancer. This study used n-butyl alcohol extract from the Litchi seed (NLS) to treat PC3 cells; results showed apoptosis induction, cell cycle arrest, decreased invasion and migration, a dose-dependent inhibition of cell growth and viability as well as inversion of epithelial to mesenchymal transition (EMT) mediated by Akt/GSK-3β. Overall findings promote the use of NLS as a safe prostate anti-cancer treatment. Lastly, a 2003 Molecular Therapy article by Galaup et al. used the PC3 xenograft model to evaluate combination therapy of angiostatin and docetaxel gene therapy. The study treated tumors with adenovirus-delivered angiostatin (AdK3) and docetaxel; results demonstrated that tumor regression was only observed with combination treatment and was correlated to a decrease in vascularization. This has clinical relevance of highlighting the importance of combination therapy in the case of these two drug targets. Studies using the PC3 cell line have provided important insights into the biology of prostate cancer and have helped identify potential targets for new therapies. PC-3 cells have been used to study the role of the AR in prostate cancer and to test new drugs that target this pathway. The PC3 cell line (human prostate) is used to create the xenograft mouse model. The PC3 xenograft model is an androgen-independent model used for preclinical assessment of tumor growth inhibition by statins (e.g. simvastatin) or chemotherapies (e.g. docetaxel, fluoropyrimidine F10).
Download Altogen Labs PC3 Xenograft Model PowerPoint Presentation: ![]()
Basic study design
- Exponential growth is maintained for PC3 cells prior to injection. Trypsinization is used to collect the cells, followed by typan blue (viability) and cell counting by flow cytometry. The suspension concentration is diluted to 10,000 cells per microliter density.
- 11-12 week old nu/nu mice receive a single s.c. injection. All injections are into the hind leg flank, and contain Matrigel plus PC3 cells in suspension.
- Tumor measurements are assessed using calipers (digital). Upon reaching averages of 100-150 mm3, animals are grouped into necessary number of treatment cohorts. In-life administration of all test compounds are performed by following the client supplied treatment schedule.
- Continual tumor measurements (daily) and whole mouse body weights are recorded.
- Final tumor measurements and euthanization occurs when tumor size reaches 2,000 mm3 or a predetermined limit per approved IACUC protocol. Excised tumors are weighed and documented (digital imaging).
- In preparation for downstream analysis, tumors/tissues are snap frozen in liquid nitrogen, stabilized (RNA-later) or prepared for histology analysis (10% NBF).
Metastatic Model
CDX models are mouse xenografts used in pre-clinical therapeutic studies. However, as primary tumors proliferate they invade surrounding tissue, become circulatory, survive in circulation, implant in foreign parenchyma and proliferate in the distant tissue. This result leads to an extremely high percentage of death in cancer patients due to metastasis. Metastatic tumor mouse models are utilized to develop novel therapeutic agents that target metastasis (anti-metastatic therapeutics).
To create a metastatic model, the cell line of interest is transfected with vectors containing green fluorescent protein (GFP) or luciferase. Maintained under antibiotic selection, only cells containing the integrated vector will survive. The new cell line clones are capable of stably expressing the gene of interest and are used in metastatic mouse model studies. Although each new cell line clone may contain its own inherent difficulties, the new cell line contains the ability to track internal tumor progression via bioluminescence (luciferase fluorescence after injecting luciferin) or fluorescence (GFP). Internal orthotopic and metastatic tumor growth (not palpable) can now be measured throughout the study, enabling a researcher to gain more insight and additional data in contrast to relying on end of study tumor weight measurements.
Case Study: U87-luc Xenograft Model
An example of Altogen Labs utilizing a luciferase expressing cell line to monitor orthotopic tumor growth is exhibited below. The same ideology of tumor observation is incorporated in metastatic tumor models.
Luciferase expressing U87-luc cells were implanted and tumors allowed to grow. Tumor growth was monitored in a Night Owl (Berthold Technologies) imaging system 10 minutes after an intraperitoneal (IP) injection of the luciferin substrate. As seen in the example below, luciferase expression (measured as photons emitted) in the U87-luc model grants the researcher a visual image and quantifiable metric for orthotopic or metastatic tumor progression.
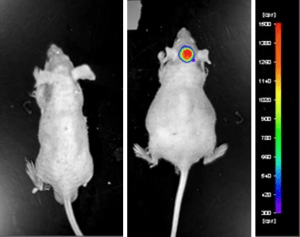
Figure 1. Luciferase expression in U87-luc orthotopic model. Control and implanted glioma mouse model fluorescence was analyzed 10 minutes after intraperitoneal luciferin injection.
View full details of the case study by clicking here.
Get Instant Quote for
PC-3 Xenograft Model
Xenograft animal models are used to assess the effectiveness of drugs against specific types of cancer. New medicines are tested on staged tumor growths that have been engrafted via subcutaneous or orthotopic inoculation in an immunocompromised mouse or rat model. All clinically approved anti-cancer agents have been evaluated with conventional nonclinical in vivo models. Xenograft studies can be highly complex, starting with the selection of the appropriate animal model, choice of tumorigenic cell line, administration method, dosing, analysis of tumor growth rates and tumor analysis (histology, mRNA and protein expression levels). Altogen Labs provides an array of laboratory services using over 90 standard CDX models and 30+ PDX models. Altogen Labs provides quantitative gene expression analysis of mRNA expression (qPCR) and protein expression analysis using the automated western blot WES system. Animal handling and maintenance at the Altogen Labs facility is IACUC-regulated and compliant to GLP standards. Following acclimation to the vivarium environment, mice are sorted according to body mass. The animals are examined daily for tumor appearance and clinical signs. We provide detailed experimental procedures, health reports and data (all-inclusive report is provided to the client that includes methods, results, discussion and raw data along with statistical analysis).
Following options are available for the PC3 xenograft model:
- PC3 Tumor Growth Delay (TGD; latency)
- PC3 Tumor Growth Inhibition (TGI)
- Dosing frequency and duration of dose administration
- Dosing route (intravenous, intratracheal, continuous infusion, intraperitoneal, intratumoral, oral gavage, topical, intramuscular, subcutaneous, intranasal, using cutting-edge micro-injection techniques and pump-controlled IV injection)
- PC3 tumor pathology / immunohistochemistry
- Alternative cell engraftment sites (orthotopic transplantation, tail vein injection and left ventricular injection for metastasis studies, injection into the mammary fat pad, intraperitoneal injection)
- Blood chemistry analysis, toxicology, and survival
- Gross necropsies and pathology / histopathology
- Positive control group employing conventional chemotherapy compound
