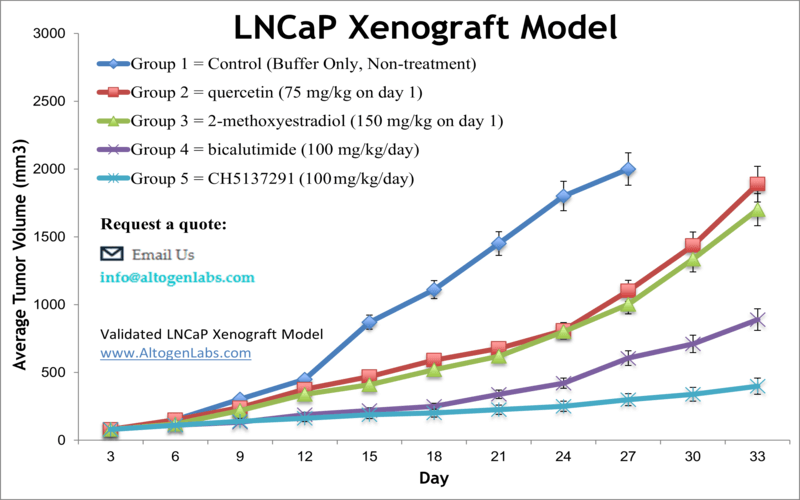
LNCaP xenograft model
Prostate cancer is the most common malignancy and the third primary cause of cancer deaths in males in the United States, as per the American Cancer Society. Xenograft preclinical studies aid in the discovery of new treatment regimens for prostate cancer patients. The LNCaP epithelial cell line was isolated from the supraclavicular lymph node metastasis of a Caucasian male in 1977. LNCaP can grow in aggregates or as a single cell. The LNCaP cell line has a high affinity for androgen receptors and is receptive to hormones. A 2015 study by Yang et al. published in PLoS One investigated if the combination of quercetin and 2-Methoxyestradiol (2-ME) could inhibit the LNCaP xenograft tumor growth in vivo. According to the article, the combination of quercetin and 2-ME blocks tumor growth in the LNCaP xenograft model and decreases side effects of quercetin or 2-ME alone, suggesting that it could be an innovative treatment approach in human prostate cancer. Lange et al. released a Clinical Cancer Research article (2012) using the LNCaP xenograft model to study aberrant glycosylation patterns and how they relate to tumor progression. Results revealed that Mgat5b appear to be biomarkers for metastatic competence and establishes β(1,6)-branched oligosaccharides as significant for prostate cancer progression; these results were also in agreement to the serum analysis of human a patients and were correlated to disease-free survival. In 2016, Byrne et al. released a study in the British Journal of Cancer evaluating the effects of androgen deprivation in LNCaP xenografts. Single-agent androgen deprivation therapy (ADT) is a prostate cancer treatment that also shows high relapse rates; results from this study revealed that ADT led to high hypoxic stress, increased VEGF related angiogenesis and upregulation of epithelial to mesenchymal transition genes. This suggests that ADT relapse may be mediated through hypoxic stress resulting in supported tumor growth and malignant progression. The LNCap cell line (human prostate) is used to create the CDX (Cell Line Derived Xenograft) LNCaP xenograft mouse model. The LNCaP xenograft model is androgen sensitive (mutated androgen receptor, AR T877A) and can be treated with modalities such as an androgen receptor antisense oligonucleotide or bicalutamide.
Download Altogen Labs LNCaP Xenograft Model PowerPoint Presentation: ![]()
Basic study design
- Cells are grown under aseptic conditions prior to collection and at exponential growth.
- LNCaP cells are then collected and viability is determined via flow cytometry, MTT, or trypan blue assay that is utilized to ensure a minimum of 98-99% viability of the cells.
- Cell suspension concentrations are adjusted such that in 150-200 µL of the Matrigel + LNCaP cell suspension there are one million cells (volume of injection is 150-200 µL). Inoculations are made into the s.c. flank of a hind leg per mouse. The mice are nu/nu and 11-12 weeks old.
- Calipers are applied for monitoring tumor growth, with tumor sizes of 100-150 mm3 needed for study initiation. Animals are then sorted into necessary groupings and client provided compounds are administered.
- Tumor measurements and mouse weights are recorded until end of study. Tumors are removed, their weights recorded and documented digitally.
- Tissue samples can be isolated for nucleic acids, submersed in RNA-later reagent, prepared for histological analysis or snap frozen in liquid nitrogen.
Get Instant Quote for
LNCaP Xenograft Model
Xenograft animal models are used to assess the effectiveness of drugs against specific types of cancer. New medicines are tested on staged tumor growths that have been engrafted via subcutaneous or orthotopic inoculation in an immunocompromised mouse or rat model. All clinically approved anti-cancer agents have been evaluated with conventional preclinical in vivo models. Xenograft studies can be highly complex, starting with the selection of the appropriate animal model, choice of tumorigenic cell line, administration method, dosing, analysis of tumor growth rates and tumor analysis (histology, mRNA and protein expression levels). Animal handling and maintenance at the Altogen Labs facility is IACUC regulated and GLP compliant. Following acclimatization to the vivarium environment, mice are sorted according to body mass. The animals are examined daily for tumor appearance and clinical signs. We provide detailed experimental procedures, health reports and data (all-inclusive report is provided to the client that includes methods, results, discussion and raw data along with statistical analysis). Additional services available include collection of tissue, histology, isolation of total protein or RNA and analysis of gene expression.
Following options are available for the LNCaP xenograft model:
- LNCaP Tumor Growth Delay (TGD; latency)
- LNCaP Tumor Growth Inhibition (TGI)
- Dosing frequency and duration of dose administration
- LNCaP tumor immunohistochemistry
- Alternative cell engraftment sites (orthotopic transplantation, tail vein injection and left ventricular injection for metastasis studies, injection into the mammary fat pad, intraperitoneal injection)
- Blood chemistry analysis
- Toxicity and survival (optional: performing a broad health observation program)
- Gross necropsies and histopathology
- Positive control group employing dox or cyclophosphamide, at a dosage of 15-20 mg/kg administered by intramuscular injection to the control group daily for the study duration
- Imaging studies: Fluorescence-based whole body imaging
