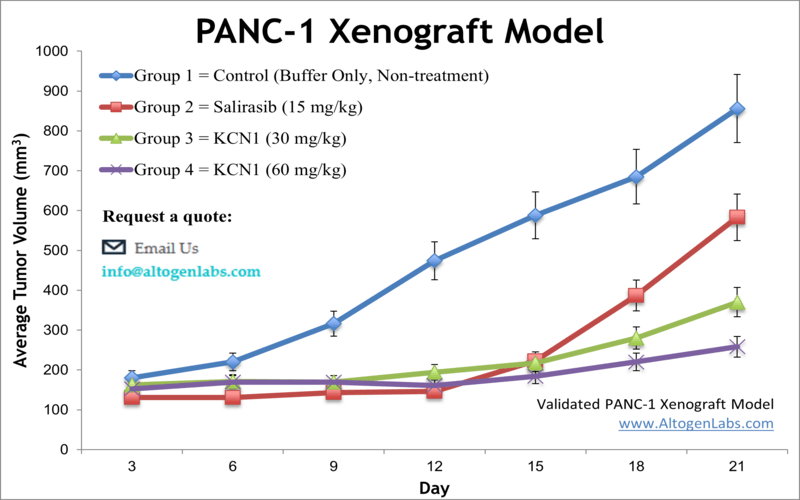
PANC1 xenograft model
PANC-1 is a pancreatic cancer cell line that is commonly used as a model system for studying pancreatic cancer and evaluating potential therapies. Pancreatic cancer is a type of cancer that arises from the cells of the pancreas, which is an organ located in the abdomen that produces enzymes and hormones that regulate blood sugar levels. Research using the PANC-1 cell line has helped to uncover important genetic and molecular changes that occur in pancreatic cancer, such as mutations in the KRAS, TP53, and CDKN2A genes. These findings have led to the development of new therapies that target these genetic alterations, such as KRAS inhibitors and immunotherapies. However, much work remains to be done to improve outcomes for patients with pancreatic cancer, and new treatment options are desperately needed. Pancreatic cancer is the fourth leading cause of cancer-related fatalities, comprising nearly 90 percent of all pancreatic malignancies. PANC-1 cells are very well studied and extensively employed as in vitro models for pancreatic ductal adenocarcinoma research. Chondroitin sulfate E (CS-E) is a highly sulfated glycosaminoglycan that promotes tumor invasion and metastasis. Sulfotransferase 15 (CHST15) is a specific enzyme that biosynthesizes CS-E. The presence of CS-E is detected in pancreatic ductal adenocarcinoma (PDAC) cells. A 2015 study by Takakura published in PLoS One investigated the effects of the CHST15 siRNA on tumor cell proliferation in vitro and growth in vivo, using the PANC-1 subcutaneous xenograft model. CHST15 siRNA significantly inhibited the expression of CHST15 mRNA in PANC-1 cells in vitro and one intratumoral injection of CHST15 siRNA almost completely blocked tumor growth. This suggests CHST15 could be a novel therapeutic option for PDAC patients. A 2013 Nanoscale Research Letters article by Li et al. used the PANC-1 model to evaluate gemcitabine-loaded albumin nanospheres, denoted GEM-ANPs, which were made by a modification to the desolvation-cross linking method. Treatment with this technique resulted in decreased tumor growth and limited toxicity, which provides a potentially clinically relevant administration technique for chemotherapy agents. Kim et al. published a 2013 article in Oncology Reports that used the PANC-1 xenograft model to evaluate the effect of introducing therapeutic stem cells expressing therapeutic genes IFN-β and HB1.F3.CD. The gene CD converts the non-toxic 5-fluorocytosine into the well-known chemotherapy agent 5-fluorouracil and INF-β is a cytokine with antitumor effects. Results demonstrated inhibition of tumor growth which supports the use of this stem cell therapy for pancreatic cancer treatment. Lastly, in 2014 Oncoscience published a study (Rak et al.) which used the Panc-1 xenograft model to evaluate T56-LIMK, an inhibitor of LIMK, a cytoskeleton regulator that inactivates cofilin and is implicated in cancer and neuronal diseases. Results reported decreased levels of cofilin phosphorylation as well as tumor growth inhibition which promotes T56-LIMK as a candidate for chemotherapy. The PANC-1 cell line (human pancreas) is used to create the cell line – derived xenograft mouse model. The PANC-1 xenograft model is a preclinical model enabling studies on tumor growth inhibition such as LIM kinases (LIMK) inhibitors (e.g. T56-LIMKi), or monotherapies (e.g. cisplatin, gemcitabine) in combination with recombinant vaccines (e.g. GLV-1h68).
Download Altogen Labs PANC1 Xenograft Model PowerPoint Presentation: ![]()
Basic study design
- To ensure high tumor take in mice, all cells are grown at exponential growth rates. The PANC-1 cells are collected via trypsin-EDTA, and viability is determined by MTT assay or trypan blue exclusion. At this point, the concentration of the cell suspension is adjusted to the required density for injection.
- One million cells (containing Matrigel plus PANC-1 cells in a vol = 100 µL) is injected subcutaneously into 10-12 week old athymic BALB/C nude mice.
- There is continual injection site monitoring to determine tumor establishment. Tumors are calipered and the in-life portion of the study begins with tumor sizes 50-100 mm3.
- Treatment cohorts are injected with test compounds according to the treatment dosing schedule. Pre-dose and post-dose tumor measurements are recorded, along with mouse body weights.
- After final dose, the mice are humanely euthanized and tissues are excised for further analysis. Tumor tissue is removed and weighed. All other tissues of interest can be snap frozen, immersed in RNAlater reagent, or placed in 10% NBF formalin for histology.
Get Instant Quote for
PANC-1 Xenograft Model
All clinically approved anti-cancer agents have been evaluated with conventional preclinical in vivo models. Xenograft studies can be highly complex, starting with the selection of the appropriate animal model, choice of tumorigenic cell line, administration method, dosing, analysis of tumor growth rates and tumor analysis (histology, mRNA and protein expression levels). Altogen Labs provides an array of laboratory services using over 90 in-house validated CDX) and over 30 PDX models. The dosing of the experimental compound of interest is initiated, for a staged study, when the mean tumor size reaches a specified volume (typically 50-100 mm3).
Animal handling and maintenance at the Altogen Labs facility is IACUC regulated and compliant to GLP. Following acclimatization to the vivarium environment, mice are sorted according to body mass. The animals are examined daily for tumor appearance and clinical signs. We provide detailed experimental procedures, health reports and data (all-inclusive report is provided to the client that includes methods, results, discussion and raw data along with statistical analysis). Additional services available include collection of tissue, histology, isolation of total protein or RNA and analysis of gene expression. Our animal facilities have the flexibility to use specialized food or water systems for inducible gene expression systems.
Following options are available for the PANC-1 xenograft model:
- PANC-1 Tumor Growth Delay (TGD; latency)
- PANC-1 Tumor Growth Inhibition (TGI)
- Dosing frequency and duration of dose administration
- Dosing route (intravenous, intratracheal, continuous infusion, intraperitoneal, intratumoral, oral gavage, topical, intramuscular, subcutaneous, intranasal, using cutting-edge micro-injection techniques and pump-controlled IV injection)
- Safety toxicology, ADME, PANC-1 tumor immunohistochemistry
- Alternative cell engraftment sites (orthotopic transplantation, tail vein injection and left ventricular injection for metastasis studies, injection into the mammary fat pad, intraperitoneal injection)
- Blood chemistry analysis
- Toxicity and survival (optional: performing a broad health observation program)
- Gross necropsies and histopathology
- Positive control group employing dox or cyclophosphamide
- Lipid distribution and metabolic assays
- Imaging studies: Fluorescence-based whole body imaging, MRI
