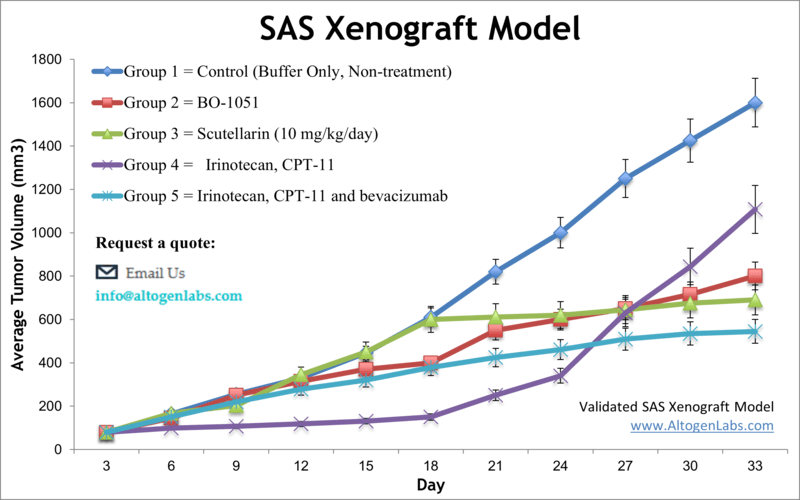
SAS xenograft model
SAS is oral cancer cell line that was established from a tongue squamous cell carcinoma in a Japanese patient. It is commonly used as a model for oral cancer research. SAS oral cancer cells are known to have mutations in the p53 and p16 genes, which are commonly altered in oral squamous cell carcinomas. These cells have been extensively studied in research to understand the underlying biology of oral cancer, test potential new treatments, and investigate the mechanisms of drug resistance. Oral cancer is frequently diagnosed at a late stage, and the outlook for complete recovery and survival is not very optimistic. However, if diagnosed early, the five-year survival for oral cancer is 75 percent versus only 20 percent for patients with late-stage diagnosis, as stated by the U.S. Department of Health and Human Services. Improvements in treatment options for late-stage patients require extensive preclinical studies that involve human tumor xenograft models. The SAS cell line was isolated from a poorly differentiated squamous cell carcinoma of the tongue. A 2013 study by Li et al. published in International Journal of Oncology examined the antitumor effects of the scutellarin on human tongue squamous carcinoma (SAS) cell line in vitro and in vivo using the SAS xenograft model. Findings reported that the scutellarin treatment significantly inhibits the growth of xenograft SAS tumors in immunodeficient mice and regulates inhibition of tumor cell proliferation, induces apoptosis and mediates expression of MMP-2, MMP-9, and integrin αvβ6 at the mRNA and protein levels in vivo. The article demonstrats the anti-tumor therapeutic effect of scutellarin by inhibition of the ability of SAS cells to metastasize. In 2015 Huang et al. published an article in BMC Cancer investigating the mechanism of honokiol, an active compound found in Magnolia officinalis, effects on eliminating cancer stem cells (CSCs) in oral cancer. Using the SAS cell model and the side population (SP) technique, results showed that hokinol treatment suppressed sphere formation in culture and xenograft growth in vivo. It was also demonstrated that hokinol treatment led to apoptosis induction and JAK2/STAT3 signaling inhibition, supporting CSC targeting for treating oral cancer. Lastly, a 2012 Integrative Cancer Therapy study (Lu et al.) used SAS cells in vitro and xenograft model to characterize the mechanism of gypenosides (Gyp), active compounds in Gynostemma pentaphyllum Makino, on tumors. Data showed that Gyp treatment caused morphological changes, cytotoxicity, cell cycle arrest, apoptosis induction (both caspase dependent and caspase independent), mitochondrial disrepair and inhibition of tumor growth in a nu/nu mouse xenograft. These results support the potential of gypenosides as anticancer agents. The SAS cell line (human tongue) is used to create the CDX (Cell Line Derived Xenograft) SAS xenograft mouse model. The SAS xenograft model exhibits anti-tumor activity after treatment with platinum-based chemotherapeutic agents, taxanes and valproic acid.
Download Altogen Labs SAS Xenograft Model PowerPoint Presentation: ![]()
Basic study design
- All flasks containing cells are maintained at a confluency allowing exponential growth. After trypsinization, cell viability and concentration are determined by flow cytometry assay. Density is adjusted such that inoculations are 1 x 106 cells in a 120-200 µL suspension (Matrigel + SAS cells suspension). Subcutaneous (s.c.), injections are performed into the hind leg of athymic BALB/C or NOD/SCID mice (10 to 12 weeks old).
- Treatment cohorts are formed after randomization of mice, which marks the beginning of the study. All test materials are injected following the client supplied dosage concentrations and schedules.
- All tumor measurements are logged (daily measurements), including mouse body weights (bi-weekly).
- The end of the study is reached at the tumor size limits (or 2,000 mm3), and necropsies are performed. Customer instructions determine the specific tissues to be collected and the method of storage (RNAlater, frozen, 10% NBF formalin, etc). Tumors are weighed; optional digital imaging is available upon request.
Get Instant Quote for
SAS Xenograft Model
The dosing of the experimental compound of interest is initiated, for a staged study, when the mean tumor size reaches a specified volume (typically 120-200 mm3). Animal handling and maintenance at the Altogen Labs facility is IACUC regulated and GLP-compliant. Following acclimation to the vivarium environment, mice are sorted according to body mass. The animals are examined daily for tumor appearance and clinical signs. We provide detailed experimental procedures, health reports and data (all-inclusive report is provided to the client that includes methods, results, discussion and raw data along with statistical analysis). Additional services available include collection of tissue, histology, isolation of total protein or RNA and analysis of gene expression. Our animal facilities have the flexibility to use specialized food or water systems for inducible gene expression systems.
Following options are available for the SAS xenograft model:
- SAS Tumor Growth Delay (TGD; latency)
- SAS Tumor Growth Inhibition (TGI)
- Dosing frequency and duration of dose administration
- Dosing route
- SAS tumor immunohistochemistry
- Blood chemistry analysis
- Toxicity and survival (optional: performing a broad health observation program)
- Gross necropsies and histopathology
- Positive control group employing dox or cyclophosphamide
- Imaging studies: Fluorescence-based whole body imaging
