A375 xenograft model (subcutaneous and metastatic)
Malignant melanoma is characterized by its highly aggressive nature and ability to metastasize to various distant sites. Resistance to BRAF inhibition is a leading cause of treatment failure for patients with BRAF-mutated metastatic melanoma. Preclinical xenograft animal models allow investigating both the tumor growth behavior and the effect of administered anticancer drugs on tumor growth dynamics. The A375 cell line was derived from skin cells of a 54-year-old female patient with malignant melanoma. A375 is a suitable host for skin cancer studies as well as human skin infections. A 2016 article by Tate et al. published in British Journal of Cancer identified an optimum dosing regimen for the abemaciclib/vemurafenib combination, using the A375 xenograft model. These findings indicate that continuous abemaciclib therapy in combination with intermittent vemurafenib offers the potential for significant tumor regression and could be a treatment strategy of choice for patients with BRAF-mutated metastatic melanoma. A 2017 Oncology Reports article (Avram et al.) used the A375 melanoma cell line to inoculate chick embryo chorioallantoic membrane (CAM) as well as Balb/c nude mice in order to develop and standardize in ovo and in vivo models for studying melanoma progression and metastasis. Results demonstrated reproducibility with standardized models and protocols in terms of metastasis and angiogenesis, supporting their use in future research. In 2015 Yang et al. released a study in the Journal of Pharmacological Sciences using the A375 xenograft model to study cisplatin resistance. Cisplatin is a well-known DNA-damaging anticancer drug and this group demonstrated that combination therapy with cisplatin and an EphB4 inhibitor successfully overcame cisplatin-resistant tumors in the xenograft models, providing a clinically relevant alternative treatment. The A375 cell line (human melanoma) is used to create the CDX (Cell Line Derived Xenograft) A375 xenograft mouse model that can be utilized for studying BRAF inhibitors (e.g. vemurafenib) as well as BCL-2 targeting.
Download Altogen Labs A375 Xenograft Model PowerPoint Presentation: ![]()
Basic study design
- A375 cells are maintained in the exponential growth phase prior to injection.
- A375 cells are then prepared for injection by trypsinization. The percentage of viable cells are determined using the trypan blue exclusion protocol (98% cell viability required).
- Each mouse (NOD/SCID, 10-12 weeks old) receive subcutaneous injections in the hind leg flank. Each injection contains one million cells of the Matrigel A375 cell suspension in a volume of 100µl
- Three time weekly, the injection sites are palpated until tumors are established. Digital calipers are used to measure tumor growth until an average size of 75-150 mm3 is reached.
- Animals are then randomized into the necessary number of treatment cohorts. Administration of the test compound is performed according to the treatment schedule.
- Tumors are measured per specified days and mouse weights are recorded 2-3 times weekly.
- When tumor size reaches the predetermined size limit or 2,000 cubic millimeters, the animals are euthanized.
- Tumors are excised from the animal, weighed and then documented by digital imaging.
- Gross necropsies are performed and specified tissues are collected for downstream analysis.
- The collected tumors and tissues can stabilized in RNA-later reagent, snap frozen in LN2, or prepared for histology.
Metastatic Model
CDX models are mouse xenografts used in pre-clinical therapeutic studies. However, as primary tumors proliferate they invade surrounding tissue, become circulatory, survive in circulation, implant in foreign parenchyma and proliferate in the distant tissue. This result leads to an extremely high percentage of death in cancer patients due to metastasis. Metastatic tumor mouse models are utilized to develop novel therapeutic agents that target metastasis (anti-metastatic therapeutics).
To create a metastatic model, the cell line of interest is transfected with vectors containing green fluorescent protein (GFP) or luciferase. Maintained under antibiotic selection, only cells containing the integrated vector will survive. The new cell line clones are capable of stably expressing the gene of interest and are used in metastatic mouse model studies. Although each new cell line clone may contain its own inherent difficulties, the new cell line contains the ability to track internal tumor progression via bioluminescence (luciferase fluorescence after injecting luciferin) or fluorescence (GFP). Internal orthotopic and metastatic tumor growth (not palpable) can now be measured throughout the study, enabling a researcher to gain more insight and additional data in contrast to relying on end of study tumor weight measurements.
Case Study: U87-luc Xenograft Model
An example of Altogen Labs utilizing a luciferase expressing cell line to monitor orthotopic tumor growth is exhibited below. The same ideology of tumor observation is incorporated in metastatic tumor models.
Luciferase expressing U87-luc cells were implanted and tumors allowed to grow. Tumor growth was monitored in a Night Owl (Berthold Technologies) imaging system 10 minutes after an intraperitoneal (IP) injection of the luciferin substrate. As seen in the example below, luciferase expression (measured as photons emitted) in the U87-luc model grants the researcher a visual image and quantifiable metric for orthotopic or metastatic tumor progression.
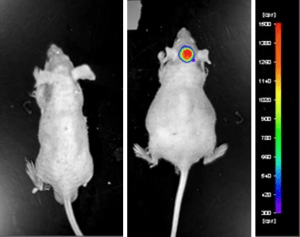
Figure 1. Luciferase expression in U87-luc orthotopic model. Control and implanted glioma mouse model fluorescence was analyzed 10 minutes after intraperitoneal luciferin injection.
View full details of the case study by clicking here.
Get Instant Quote for
A375 Xenograft Model
Xenograft animal models are used to assess the effectiveness of drugs against specific types of cancer. New medicines are tested on staged tumor growths that have been engrafted via subcutaneous or orthotopic inoculation in an immunocompromised mouse or rat model. All clinically approved anti-cancer agents have been evaluated with conventional preclinical in vivo models. Xenograft studies can be highly complex, starting with the selection of the appropriate animal model, choice of tumorigenic cell line, administration method, dosing, analysis of tumor growth rates and tumor analysis (histology, mRNA and protein expression levels). Animal handling and maintenance at the Altogen Labs facility is IACUC regulated and GLP compliant. Following acclimation to the vivarium environment, mice are sorted according to body mass. The animals are examined daily for tumor appearance and clinical signs. We provide detailed experimental procedures, health reports and data (all-inclusive report is provided to the client that includes methods, results, discussion and raw data along with statistical analysis). Additional services available include collection of tissue, histology, isolation of total protein or RNA and analysis of gene expression. Our animal facilities have the flexibility to use specialized food or water systems for inducible gene expression systems.
Following options are available for the A375 xenograft model:
- A375 Tumor Growth Delay (TGD; latency)
- A375 Tumor Growth Inhibition (TGI)
- Dosing frequency and duration of dose administration
- Dosing route
- A375 tumor immunohistochemistry
- Alternative cell engraftment sites (orthotopic transplantation, tail vein injection and left ventricular injection for metastasis studies, injection into the mammary fat pad, intraperitoneal injection)
- Blood chemistry analysis
- Toxicity and survival
- Gross necropsies and histopathology
- Positive control group employing cyclophosphamide or cisplatin, at a dosage of 15-25 mg/kg
