NCI-H1155 xenograft model
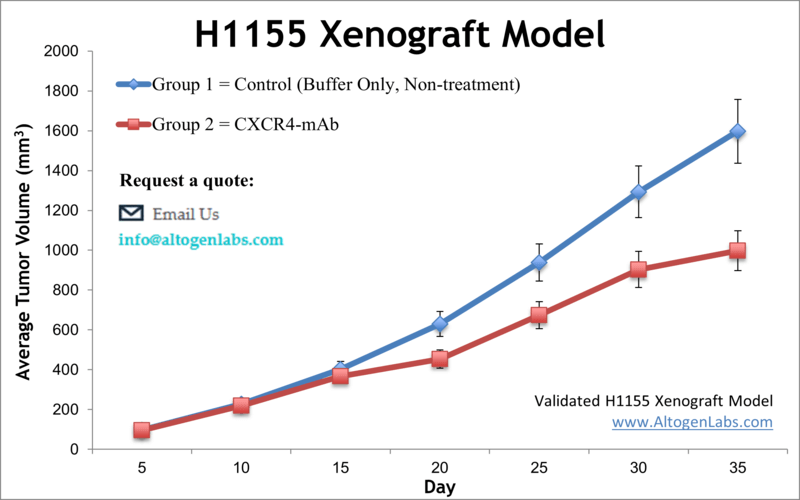
NCI-H1155 is a non-small cell lung cancer cell line that was derived from the lung of a patient with a poorly differentiated carcinoma. The NCI prefix indicates that this cell line was developed and is maintained by the National Cancer Institute (NCI) in the United States. NCI-H1155 cells have been extensively used in cancer research to study various aspects of lung cancer biology, including the molecular mechanisms underlying tumor initiation, progression, and drug resistance. Lung cancer is the second most common malignancy worldwide, despite the highly preventable nature. It accounts for more fatalities than colon, breast, and prostate cancers combined, as per the Centers for Disease Control and Prevention. Therefore, preclinical research tools, particularly xenotransplantation of tumor cells into immunodeficient mice, are essential for patients and could aid in assessing the effectiveness of drugs against lung cancer. The H1155 epithelial cell line was derived from a lymph node metastasis obtained from a 36-year-old Caucasian male patient with lung carcinoma before the treatment. A 2008 study by Wang et al. published in Cancer Research investigated the efficacy and mechanisms of the combination of gefitinib or erlotinib with OSU-03012, a celecoxib-derived antitumor agent, to overcome EGFR inhibitor resistance in the H1155 cell line. The article indicates that the OSU-03012/EGFR inhibitor combination induces apoptosis in H1155 cells, suggesting a link between drug sensitivity and basal phospho-Akt levels independently of EGFR expression status. Moreover, in vivo suppression of tumor growth was observed in the H-1155 tumor xenograft model in immunocompromised mice. These findings demonstrate a new approach to overcome EGFR inhibitor resistance in non-small cell lung cancer, using the OSU-03012/EGFR inhibitor combination for the modulation of Akt and ER stress pathways. Lei et al. released an Oncotarget study (2017) investigating the effect of overexpressing erythropoietin (EPO) with its receptor (EPOR) and reported that in the H1155 xenograft non-small cell lung cancer (NSCLC) model EPO/EPOR overexpression is associated with increased growth and proliferation (via cell cycle promotion with Jak2/Stat5/cyclinD1) and siRNA interference with EPOR reversed this effect and prolonged survival. This signaling pathway was also demonstrated to mediate hypoxia induced growth; together data suggest blocking EPO/EPOR as a therapeutic strategy. A 2016 Oncotarget study by Chatterjee et al. used the H1155 model to demonstrate the use of a humanized antibody against the PD-L1 checkpoint ligand for imaging its expression. It is thought that PD-L1 expression may be a biomarker for the success of checkpoint blockade therapy. Results using near-infrared dye conjugated NIR-PD-Li-mAb MPDL3280A produced successful imaging in mouse xenografts, which has potential for clinical translation for optical imaging and personal medicine. On a similar note, the 2014 Oncotarget study by Azad et al. used the H1155 to demonstrate the use of an antibody against CXCR4 for molecular imaging and predictive therapeutic efficacy. The CXC chemokine receptor 4 (CXCR4) is overexpressed in tumors and contributes to tumor progression, growth and metastasis and so the group used a Zirconium-89 labeled monoclonal antibody (89Zr-CXCR4-mAB) that successfully detected expression levels of CXCR4 in mouse xenografts which has potential for selecting for patients that would best respond to CXCR4 inhibition therapy. The H1155 cell line (human lung) is used to create the CDX (Cell Line Derived Xenograft) H1155 xenograft mouse model. The H1155 xenograft model enables efficacy studies for acquired or preexisting EGFR-inhibitor resistance.
Download Altogen Labs H1155 Xenograft Model PowerPoint Presentation: ![]()
H1155 Lung Cancer Xenograft Model: Download ![]()
Studying NSCLC Progression and Therapy in an Orthotopic Model
The orthotopic H1155 xenograft model involves the implantation of H1155 tumor cells directly into the lungs of immunocompromised mice, providing a physiologically relevant environment for studying non-small cell lung cancer (NSCLC). This model closely mimics the tumor microenvironment, allowing researchers to investigate key aspects of tumor progression, invasion, and metastasis. Unlike subcutaneous models, orthotopic implantation enables the evaluation of lung-specific interactions, including responses to targeted therapies and immunotherapies. Advanced imaging techniques, such as bioluminescence or PET-CT, are often used to monitor tumor growth non-invasively. The model is particularly useful for studying mechanisms of metastasis and drug resistance in NSCLC, offering valuable insights into disease progression.
H1155 Gene Expression and Tumor Biology Insights
H1155 belongs to a group of NSCLC cell lines that do not induce lymphatic vessel formation, distinguishing it from lymphangiogenic counterparts, such as HCC827 or H1993. Genome-wide mRNA expression analysis also revealed significant differences in gene expression between lymphangiogenic and non-lymphangiogenic NSCLC cell lines, with vascular endothelial growth factor C (VEGF-C) identified as a key regulator of lymphangiogenesis. Notably, VEGF-C expression was approximately 50-fold lower in the non-lymphangiogenic group, which includes the H1155 cell, which has researchers suggesting that the H1155 cell line may exhibit distinct tumor progression and possible metastatic behaviors.
Taxol Resistance in H1155 Lung Cancer Cells
The H1155 cell line has been extensively studied for its response to taxol, a chemotherapeutic agent that targets microtubules. Research has identified several taxol-sensitizer genes that enhance the cytotoxic effects of taxol when silenced in H1155 cells. These genes were shown to mediate resistance mechanisms, as their knockdown led to a significant decrease in cell viability following taxol treatment. However, further investigation revealed that these sensitization effects were largely specific to H1155 and did not translate to other NSCLC or cancer cell lines, highlighting the unique genetic dependencies of this model. Additionally, overexpression of these genes was observed in taxol-resistant ovarian cancer cells, suggesting a potential role in acquired drug resistance. These findings underscore the importance of H1155 as a valuable tool for studying taxol response and chemoresistance mechanisms in lung cancer.
HCC1155 Subculturing Protocols and Handling Procedures
To ensure optimal cell viability, researchers thawed the H1155 cell vial and initiated culture as soon as it was received. If immediate culture initiation was not possible, the frozen culture was stored in the liquid nitrogen vapor phase, as storage at -70°C would have compromised cell viability. Thawing was carried out by gently agitating the vial in a 37°C water bath for approximately 2 minutes, while taking care to avoid contact between the O-ring and cap with the water to prevent contamination. Once thawed, the vial was removed from the water bath and decontaminated with a 70% ethanol solution. All subsequent procedures were performed under strict aseptic conditions. The thawed cell suspension was transferred into a centrifuge tube containing 9.0 mL of complete culture medium and centrifuged at 125 x g for 5 to 7 minutes. After centrifugation, the cell pellet was resuspended in the recommended culture medium, with careful attention to avoid excessive alkalinity. The suspension was then dispensed into a 25 cm² culture flask, which had been pre-warmed in the incubator for at least 15 minutes to ensure the medium reached a pH of 7.0 to 7.6. Finally, the culture was incubated at 37°C in a 5% CO2 atmosphere to support optimal cell growth. Researchers maintained cultures by adding fresh medium or replacing the existing medium as needed. When necessary, they centrifuged the cell suspension and resuspended it in fresh medium to establish or propagate cultures. As cell density increased, additional medium was added to support optimal growth. Medium was renewed every 2 to 3 days to ensure the cells had adequate nutrients for sustained culture.
H1155 Gene Expression and Tumor Biology Insights
H1155 belongs to a group of NSCLC cell lines that do not induce lymphatic vessel formation, distinguishing it from lymphangiogenic counterparts, such as HCC827 or H1993. Genome-wide mRNA expression analysis also revealed significant differences in gene expression between lymphangiogenic and non-lymphangiogenic NSCLC cell lines, with vascular endothelial growth factor C (VEGF-C) identified as a key regulator of lymphangiogenesis. Notably, VEGF-C expression was approximately 50-fold lower in the non-lymphangiogenic group, which includes the H1155 cell, which has researchers suggesting that the H1155 cell line may exhibit distinct tumor progression and possible metastatic behaviors.
Basic study design
1. Cell growth is maintained at conditions of exponential growth prior to injection. H1155 cells are collected by trypsinization, and then cell viability is determined using a trypan blue exclusion test, with a minimum of 99% cell viability required. The cell suspension is then adjusted prior to inoculation.
2. NOD/SCID or athymic BALB/C mice (11-12 weeks) receive a subcutaneous (s.c.) single injection in the flank of the hind leg containing 1 million cells (120-160 µL volume) of the matrigel-H1155 cell suspension.
3. To determine tumor establishment, injection sites are palpated up to three times a week. When tumors are established, they are measured via digital calipers and monitored until they reach 50-150 mm3.
4. The mice are randomized into predetermined treatment cohorts, and the compound of interest is administered according to the study design treatment schedule.
5. All tumors are measured on a daily basis, with mouse weights recorded at least 3 times a week.
6. As tumor size reaches 2,000 mm3 (or the predetermined limit by approved IACUC protocol), animals are euthanized.
7. The tissue collection and necropsy are performed as defined in the termination of the experiment.
8. Tumor excision, weight and digital imaging data are documented.
9. A standard gross necropsy is performed and designated tissues are collected.
10. Altogen Labs can snap freeze the tumors/tissues, submerse in RNA-later reagent, prepare for histology or isolate nucleic acid for genetic analysis.
Get Instant Quote for
H1155 Xenograft Model
Xenograft animal models are used to assess the effectiveness of drugs against specific types of cancer. New medicines are tested on staged tumor growths that have been engrafted via subcutaneous or orthotopic inoculation in an immunocompromised mouse or rat model. All clinically approved anti-cancer agents have been evaluated with conventional preclinical in vivo models. Xenograft studies can be highly complex, starting with the selection of the appropriate animal model, choice of tumorigenic cell line, administration method, dosing, analysis of tumor growth rates and tumor analysis (histology, mRNA and protein expression levels).Researchers investigating the role of specific proteins or gene products in regulating tumor growth can benefit from development of protein overexpression (genetically engineered to ectopically express proteins, tumor suppressors, or oncogenes) and RNAi cell lines with long term gene silencing. Altogen Labs provides quantitative gene expression analysis of mRNA expression (RT-PCR) and protein expression analysis using the WES system (ProteinSimple). Animal handling and maintenance at the Altogen Labs facility is IACUC regulated and GLP compliant. Following acclimatization to the vivarium environment, mice are sorted according to body mass. The animals are examined daily for tumor appearance and clinical signs. We provide detailed experimental procedures, health reports and data (all-inclusive report is provided to the client that includes methods, results, discussion and raw data along with statistical analysis). Additional services available include collection of tissue, histology, isolation of total protein or RNA and analysis of gene expression.
Following options are available for the H1155 xenograft model:
- H1155 Tumor Growth Delay (TGD; latency)
- H1155 Tumor Growth Inhibition (TGI)
- Dosing frequency and duration of dose administration
- Dosing route (intravenous, intratracheal, continuous infusion, intraperitoneal, intratumoral, oral gavage, topical, intramuscular, subcutaneous, intranasal, using cutting-edge micro-injection techniques and pump-controlled IV injection)
- H1155 tumor immunohistochemistry
- Alternative cell engraftment sites (orthotopic transplantation, tail vein injection and left ventricular injection for metastasis studies, injection into the mammary fat pad, intraperitoneal injection)
- Safety toxicology, ADME, and blood chemistry analysis
- Toxicity and survival (optional: performing a broad health observation program)
- Gross necropsies and histopathology
- Positive control group employing cyclophosphamide, at a dosage of 30-50 mg/kg
- Imaging studies: Fluorescence-based whole body imaging
