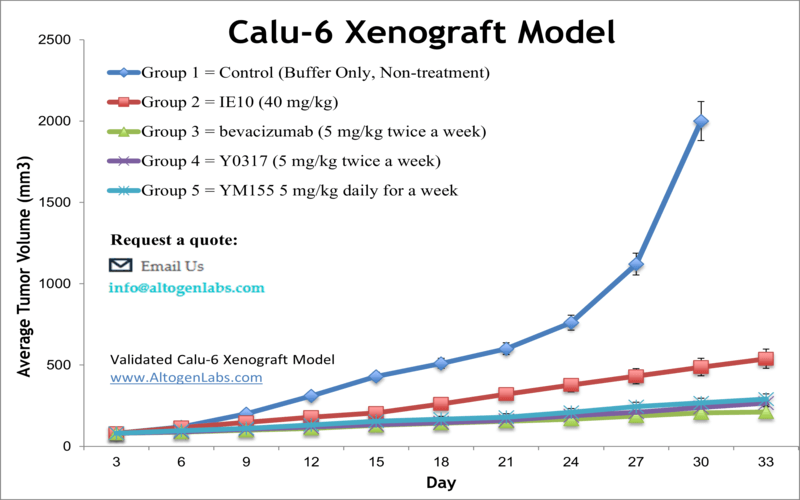
Calu-6 xenograft model
Lung cancer is the leading cause of cancer-related death worldwide among both women and men. It affects smokers 15 to 30 times more often than people who do not smoke and is the most preventable cancer, per the American Cancer Society. Calu-6 cancer xenografts are used to test the efficacy of various treatment modalities, such as chemotherapy, radiation therapy, and immunotherapy, for the treatment of lung cancer. The Calu-6 epithelial cell line is isolated from lung tissue of a 61-year-old Caucasian female with anaplastic carcinoma and is a suitable host for lung cancer research. Calu6 cells are instrumental in the development of safer and more efficient inhaled therapeutics. A 2016 study published in Oncotarget demonstrates that an increase in the levels of G protein-coupled receptor (GPR171) is vital for lung cancer tumor progression both in vitro and in vivo. The study indicates that anti-GPR171 antibody treatment inhibits proliferation of lung carcinoma cells in the Calu-6 xenograft model, indicating that GPR171 is a promising target for the development of antineoplastic drugs. A 2007 Molecular Cancer Therapeutics study by Smith et al. used the Calu-6 mouse xenograft model to study the effect of AZD2171, an inhibitor of endothelial growth factor (EGFR) signaling. Data demonstrated decreased tumor microvasculature, decreased phosphorylation of VEGFR-2 and decreased tumor growth with AZD2171 treatment; the study also used phospho-specific antibodies (pY1175/1173 and pY1214/1212) that are suggested to have potential as a pharmacodynamics marker of activation of VEGFR-2. Jiang et al. published a 2015 study using Calu-6 xenografts to investigate the effect of hypoxia on radiosensitivity mediated by the poly(ADP-ribose) polymerase (PARP) inhibitor olaparib. Results suggested that hypoxia enhances the effect of olaparib-mediated radiosensitivity by causing “contextual synthetic killing” which refers to how a chronically hypoxic microenviconment results in suppression of homologous recombination and protein expression in tumor cells. Lastly, Coxon et al. released a Molecular Cancer study (2015) using the Calu-6 xenograft model to test the combination-therapeutic effects of motesanib, a selective vascular endothelial growth factor receptor (VEGF) -1, -2, -3, Kit and platelet-derived growth factor receptor agonist, with cisplatin or docetaxel in non-small cell lung cancer (NSCLC) models with diverse genetic mutations. The group concluded that motesanib enhanced both cisplatin and docetaxel antitumor effects, which are primarily anti-angiogenesis mediated, in NSCLC models, providing potential clinical relevance. The Calu-6 cell line (human lung) is used to create the CDX (Cell Line Derived Xenograft) Calu-6 xenograft mouse model. The Calu-6 tumor model is an established xenograft model used to study the anti-tumor activity of therapeutics, such as bevacizumab, docetaxel, cisplatin and motesanib.
Calu-6 Xenograft Model: Download ![]()
Download Altogen Labs Calu6 Xenograft Model PowerPoint Presentation: ![]()
Calu-6 cell line
The Calu-6 cell line is a valuable model for studying the oncogenic characteristics of lung cancer, particularly in relation to key oncogenes such as KRAS. KRAS mutations, commonly associated with non-small cell lung cancer (NSCLC), are present in Calu-6 cells and contribute to the cell line’s aggressive tumorigenic behavior, including enhanced cell proliferation, survival, and resistance to apoptosis. These characteristics make the Calu-6 cell line an ideal platform for investigating the molecular pathways driving lung cancer and evaluating potential therapeutic targets. Additionally, Calu-6 allows for the assessment of the effects of oncogene-targeted therapies, providing critical insights into treatment efficacy and resistance mechanisms. By studying the oncogenic properties of Calu-6, researchers can gain a deeper understanding of the molecular underpinnings of lung cancer and explore novel strategies for targeted treatment.
Basic study design
- Calu-6 cells used for injection are maintained under conditions of exponential growth prior to injection
2. Calu-6 cells are prepared for injection using trypsinization and counting viable cells using a trypan blue exclusion test (99% cell viability required). Cell suspension is adjusted to appropriate density.
3. Each mouse (athymic BALB/C (nu/nu), 11-12 w.o.) receives a single subcutaneous injection in a single hind leg. Each injection contains one million cells in a volume of 100-150 µL of the Matrigel/Calu-6 cell suspension.
4. Injection sites are palpated three times weekly until tumors are established. Tumors are measured via digital calipers until they reach an average size of 100-120 mm3.
5. Animals are randomized into predetermined treatment cohorts and the administration of the compound of interest is performed according to the test article treatment schedule.
6. Tumors are then measured daily and the mouse weights are recorded up to 2-3 times a week.
7. Animals are then euthanized when tumor size reaches the predetermined size limit (or approximately 2,000 cubic millimeters).
8. A necropsy is performed as defined in the agreed upon termination of experiment.
9. Tumors are excised from the mice, weighed and then documented by digital imaging.
10. Tissues are collected by a standard gross necropsy for downstream analysis.
11. Tumors/tissues can be immersed in RNAlater reagent for stabilization, snap frozen in liquid nitrogen (LN2) or prepared for histology.
Get Instant Quote for
Calu-6 Xenograft Model
Case Study: Response to Cediranib in Calu-6 NSCLC Xenografts
In a study conducted by Jiang Y et al., published by Lung Cancer journal, researchers investigated the acute vascular response to the VEGFR tyrosine kinase inhibitor cediranib in non-small-cell lung cancer (NSCLC) xenografts with distinct tumor stromal architectures. Two xenograft models were employed: Calu-3 (representing the stromal vessel phenotype) and Calu-6 (representing the tumor vessel phenotype). The results demonstrated that cediranib markedly reduced tumor perfusion and induced hypoxia in Calu-3 xenografts but had minimal effects on Calu-6 tumors. These differences highlight the sensitivity of stromal vessel phenotypes to cediranib treatment compared to tumor vessel phenotypes. Moreover, Calu-3 xenografts exhibited significant tumor regression following treatment, whereas Calu-6 tumors only showed a trend toward growth inhibition. This study emphasizes the potential of tumor stromal architecture as a predictor of response to VEGFR TKI therapy, with Calu-6 serving as a critical model for investigating the less responsive tumor vessel phenotype.
Efficacy of Motesanib in Calu-6 NSCLC Xenografts
In another study done by Coxon A et al., published in Molecular Cancer journal, researchers explored the efficacy of motesanib, a VEGF receptor antagonist, in inhibiting tumor growth in five human non-small-cell lung cancer (NSCLC) xenograft models, including the Calu-6 model. Motesanib alone demonstrated dose-dependent tumor growth inhibition across all models, but Calu-6 tumors were notably resistant, requiring the highest tested dose to achieve significant inhibition. This resistance highlights the unique biology of Calu-6, which represents an aggressive NSCLC subtype with reduced sensitivity to single-agent angiogenesis inhibitors. However, when combined with standard chemotherapy agents like cisplatin, motesanib significantly enhanced tumor growth suppression in Calu-6 and other models, suggesting a synergistic effect. The study attributed motesanib’s antitumor activity to its antiangiogenic mechanisms, targeting tumor vasculature rather than tumor cells directly.
Xenograft animal models are used to assess the effectiveness of experimental test compounds against specific types of cancer. New medicines are tested on staged tumor growths that have been engrafted via subcutaneous or orthotopic inoculation in an immunocompromised mouse or rat model. All clinically approved anti-cancer agents have been evaluated with conventional preclinical in vivo models. Xenograft studies can be highly complex, starting with the selection of the appropriate animal model, choice of tumorigenic cell line, administration method, dosing, analysis of tumor growth rates and tumor analysis (histology, mRNA and protein expression levels). Animal handling and maintenance at the Altogen Labs facility is regulated by IACUC and GLP-compliant. Following acclimatization to the vivarium environment, mice are sorted according to body mass. The animals are examined daily for tumor appearance and clinical signs. We provide detailed experimental procedures, health reports and data (all-inclusive report is provided to the client that includes methods, results, discussion and raw data along with statistical analysis).
Following options are available for the Calu-6 xenograft model:
- Calu-6 Tumor Growth Delay (TGD; latency)
- Calu-6 Tumor Growth Inhibition (TGI)
- Dosing frequency and duration of dose administration
- Dosing route (intravenous, intratracheal, continuous infusion, intraperitoneal, intratumoral, oral gavage, topical, intramuscular, subcutaneous, intranasal, using cutting-edge micro-injection techniques and pump-controlled IV injection)
- Calu-6 tumor pathology and immunohistochemistry
- Alternative cell engraftment sites (orthotopic transplantation, tail vein injection and left ventricular injection for metastasis studies, injection into the mammary fat pad, intraperitoneal injection)
- ADME, safety toxicology, genotox, blood chemistry analysis, etc
- Toxicity and survival (optional: performing a broad health observation program)
- Positive control group employing known chemotherapy drugs can be administered by intramuscular injection to the control group daily for the study duration
- Imaging studies: Fluorescence-based whole body imaging
