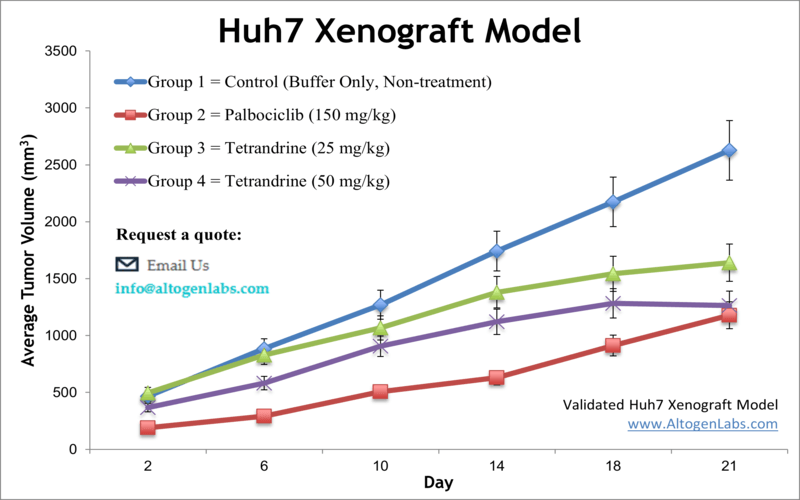
Huh-7 xenograft model
Huh7 cells are notable for their ability to produce infectious hepatitis C virus particles in vitro, which makes them a valuable tool for studying the biology of hepatitis C virus infection and for evaluating the effectiveness of new anti-viral drugs. Researchers also use Huh7 cells to investigate the molecular mechanisms underlying liver cancer development and progression, and to identify potential therapeutic targets and biomarkers of the disease. Hepatocellular carcinoma (HCC) is one of the deadliest malignancies worldwide, and innovative treatments are urgently needed. Xenograft rodent models that mimic different subclasses of neoplasm are instrumental in the evaluation of therapeutic agents targeting specific molecular pathways. The Huh7 cell line was established from a liver tumor of a 57-year-old Japanese male patient in 1985. Huh7 is frequently utilized in liver cancer research and studies on iron metabolism. A 2009 study published in Journal of Hepatology, investigated the potential of an Aurora kinase inhibitor, VE-465, for targeted therapy of HCC using the Huh7 xenograft model. The study demonstrates that VE-465 suppresses Aurora kinase activity, inducing apoptosis in the Huh7 xenograft model and preventing tumor growth supporting further clinical trials looking into the anticancer effect in human HCC. Chi et al. released a 2014 study demonstrating the antitumor activity of adenovirus mediated artificial microRNAs targeting survivin, which is highly expressed in fetal tissue and tumors and supports cell division while suppressing apoptosis. Cells treated with artificial miRNAs demonstrated increased apoptosis, caspase 3 levels, cleaved poly (ADP-ribose) polymerase, activation of p53 and an increase in cell cycle arrest. These results support the targeting of survivin, which is absent in terminally differentiated tissue, in HCC. A last example is a 2015 Nature study where Zhuo et al. used a Huh7 model for an in vitro and in vivo study demonstrating the mechanism of action of ailanthone, a component of the traditional Chinese medicine Ailanthus altissima. Data showed that treatment with ailanthone caused cell cycle arrest (observed via cyclin, CDK, p27 and p21 levels), ATM/ATR mediated DNA damage, increased levels of proapoptotic proteins (PARP and caspase cleavage) as well as inhibition of angiogenesis and tumor growth in xenografts. Overall this promotes ailanthone as a potential anticancer treatment. The Huh7 cell line (human liver) is used to create the CDX (Cell Line Derived Xenograft) Huh7 xenograft mouse model. The Huh-7 xenograft model of HCC (human hepatocellular carcinoma) is a preclinical mouse model that enables tumor growth inhibition studies targeting FGFR4, kinase inhibitors (e.g. BZG-4000), anti-EGFRvIII antibodies or other anti-tumor growth therapeutics (e.g. sorafenib, silibinin).
Download Altogen Labs Huh7 Xenograft Model PowerPoint Presentation: ![]()
Basic study design
- Exponential growth of Huh7 cells is maintained prior to the start of the study. High cell viability is required to proceed with injections into mice. Viability and cell count is determined via flow cytometry (Guava), MTT, and trypan blue assays.
- One million cells (injection volume = 100 – 200 µL) of the Huh7 + Matrigel suspension is injected subcutaneously (s.c.) into the flank of one hind leg. The mice used in the study can be 10 to 12 week old NOD/SCID or athymic BALB/C.
- Injection sites are continually examined for tumor growth until tumors reach 50-150 mm3. Mice are sorted into study groups, and then the compounds of interest are injected following the dosing schedule.
- Tumor measurements (daily) and mouse weights (2-3 times weekly) are logged.
- The end of the study is marked when the tumor size limit is reached (or 2,000 mm3), and the mice are humanely euthanized.
- As noted in the experimental design, necropsies and tissue collections are performed. Tumor resections, weights and digital images are documented. Remaining tissues are collected for downstream analysis. All collected tumors/tissues can be snap frozen, immersed in RNA-later reagent, nucleic acids isolated or fixed in 10% NBF formalin for histological analysis.
Get Instant Quote for
Huh7 Xenograft Model
Xenograft animal models are used to assess the effectiveness of drugs against specific types of cancer. New medicines are tested on staged tumor growths that have been engrafted via subcutaneous or orthotopic inoculation in an immunocompromised mouse or rat model. All clinically approved anti-cancer agents have been evaluated with conventional preclinical in vivo models. Xenograft studies can be highly complex, starting with the selection of the appropriate animal model, choice of tumorigenic cell line, administration method, dosing, analysis of tumor growth rates and tumor analysis (histology, mRNA and protein expression levels). Animal handling and maintenance at the Altogen Labs facilities are IACUC regulated and GLP compliant.
Following options are available for the Huh-7 xenograft model:
- Huh7 Tumor Growth Delay (TGD; latency)
- Huh7 Tumor Growth Inhibition (TGI)
- Dosing frequency and duration of dose administration
- Dosing route (intravenous, intratracheal, continuous infusion, intraperitoneal, intratumoral, oral gavage, topical, intramuscular, subcutaneous, intranasal, using cutting-edge micro-injection techniques and pump-controlled IV injection)
- Huh7 tumor immunohistochemistry
- Alternative cell engraftment sites (orthotopic transplantation, tail vein injection and left ventricular injection for metastasis studies, injection into the mammary fat pad, intraperitoneal injection)
- Blood chemistry analysis
- Safety Toxicology
- Gross necropsies and histopathology
- Positive control group employing cyclophosphamide, at a dosage of 10-25 mg/kg
- Imaging studies: Fluorescence-based whole body imaging
