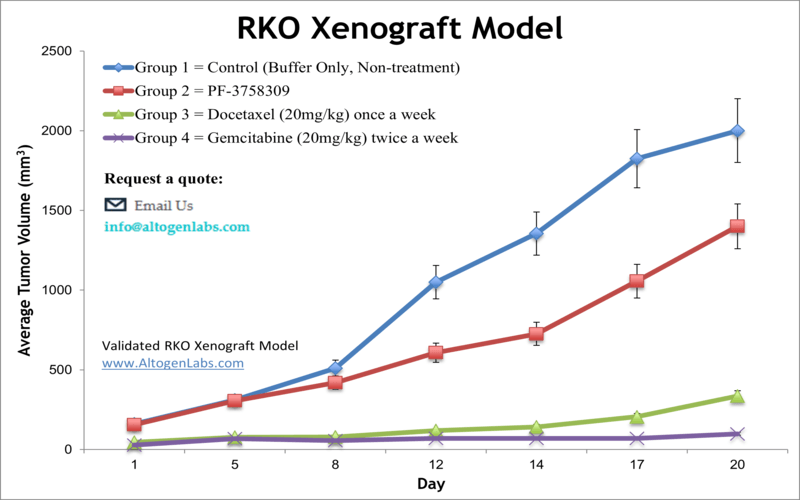
RKO xenograft model
The RKO cell line is a human colorectal carcinoma line originally derived from a 63-year-old male patient and is widely utilized in oncology research due to its well-defined genetic background and reliable tumorigenicity in vivo. Characterized by its poorly differentiated epithelial morphology, RKO cells retain wild-type p53 function but lack expression of the human thyroid hormone receptor beta (h-TRβ). This cell line expresses high levels of the urokinase-type plasminogen activator receptor (u-PAR), which is associated with tumor cell invasion and extracellular matrix remodeling. RKO cells form aggressive tumors in immunodeficient murine hosts, making them particularly useful in xenograft-based drug development studies. Their short doubling time and stable genomic profile support their application in investigations of gene silencing, signal transduction, and chemotherapeutic resistance. Recent studies have leveraged the RKO xenograft model to explore molecular targets and combination treatment strategies in colorectal cancer. For instance, small RNA interference targeting the NOB1 gene demonstrated significant tumor growth suppression, mediated through alterations in pro-apoptotic BAX and WNT signaling, indicating the involvement of NOB1 in tumor cell proliferation, apoptosis, and angiogenesis regulation. In another study evaluating the clinical relevance of BRAF inhibitors, RKO cells, which exhibit de novo resistance to vemurafenib, served as a model for testing combination therapies. The addition of cetuximab, irinotecan, capecitabine, or bevacizumab enhanced antitumor efficacy, suggesting that resistance to BRAF inhibition can be overcome through multi-agent therapeutic strategies. Furthermore, the role of hypoxia-inducible factor 1-alpha (HIF-1α) in nonhypoxic tumor growth was assessed using the RKO xenograft model, where HIF-1α was shown to support proliferation independently of hypoxia, while VEGF inhibition delayed tumor growth by expanding hypoxic tumor regions. These findings underscore the value of the RKO model in dissecting molecular determinants of drug response and tumor adaptation. RKO xenografts are frequently used to evaluate both targeted therapies and immunotherapies, including agents such as pembrolizumab and ipilimumab, making them a versatile platform for preclinical efficacy assessment in colorectal cancer research.
RKO Colon Cancer CRC Orthotopic And Subcutaneous Xenograft Model: Download ![]()
Download Altogen Labs RKO Xenograft Model PowerPoint Presentation: ![]()
RKO Cell Line
The RKO cell line, established from a poorly differentiated human colorectal carcinoma, is a widely studied model characterized by microsatellite instability (MSI-H), wild-type TP53, and the absence of common KRAS and BRAF mutations. These genetic attributes, combined with a hypermethylated epigenome and silencing of key DNA mismatch repair genes such as MLH1, position RKO cells as a valuable tool for investigating epigenetic regulation, genomic instability, and p53-dependent apoptotic pathways in colorectal cancer. RKO cells exhibit robust responses to DNA-damaging agents and are frequently employed to evaluate the efficacy of chemotherapeutics, particularly those targeting the p53 axis or exploiting MSI-associated vulnerabilities. Additionally, they have been used in studies exploring the reversal of gene silencing via DNA methyltransferase inhibitors, revealing potential for sensitization to immunotherapies through reactivation of immune signaling pathways. Despite their extensive use, gaps remain in understanding how epigenetic modulation influences immune evasion and how MSI-H status affects tumor-immune dynamics in physiologically relevant models.
Subcutaneous RKO Xenografts in Colorectal Cancer Research
Subcutaneous xenograft transplantation is a widely employed method in preclinical oncology, enabling consistent and measurable tumor growth through the implantation of human cancer cells into the subcutaneous tissue of immunodeficient mice. This approach facilitates the evaluation of therapeutic efficacy, tumor progression, and molecular responses within a controlled in vivo environment. The RKO colorectal cancer cell line has proven especially valuable in this context due to its microsatellite instability-high (MSI-H) status, intact TP53, and absence of common KRAS and BRAF mutations. These characteristics reflect a distinct molecular subtype of colorectal cancer and support the use of RKO xenografts for studying DNA mismatch repair deficiencies, p53-mediated responses, and epigenetic regulation. RKO tumors consistently engraft and proliferate in vivo, replicating key histopathological features of poorly differentiated colorectal carcinomas, and exhibit pronounced sensitivity to DNA-damaging agents, offering a relevant model for evaluating genotoxic therapies.
In recent research, subcutaneous RKO xenografts have also been utilized to investigate the effects of epigenetic therapies and immune modulators. DNA methyltransferase inhibitors, for example, have been shown to reactivate silenced immune-related genes, potentially enhancing tumor immunogenicity. However, the immunodeficient status of host animals limits direct assessment of immune-tumor interactions, necessitating additional models for immunotherapy studies. Despite this constraint, RKO xenografts remain a reliable platform for analyzing tumor behavior under defined genetic conditions, advancing the understanding of treatment resistance, and enabling targeted drug development. Their reproducibility and molecular specificity continue to support their relevance in colorectal cancer research and contribute to the broader goals of precision medicine.
Orthotopic RKO Models in Colorectal Cancer Research
Orthotopic xenograft transplantation has emerged as a superior model for studying colorectal cancer, offering an anatomically and biologically relevant environment that closely mimics the tumor’s native site. In this approach, human cancer cells are implanted directly into the colonic or rectal wall of immunodeficient mice, allowing for the evaluation of tumor growth, local invasion, and the initiation of metastasis within a context that reflects physiological conditions. RKO cells, which exhibit microsatellite instability, an intact TP53 gene, and epigenetic silencing of MLH1, have been successfully used in orthotopic models, particularly when engineered with luciferase reporters for non-invasive imaging. These models have demonstrated consistent tumor engraftment, progressive disease, and metastasis to clinically relevant organs such as the liver, pancreas, and small intestine, thereby validating their application in studies of tumor dissemination and microenvironmental interactions.
The orthotopic RKO model provides a platform to investigate critical aspects of colorectal cancer progression, including stromal remodeling, peritoneal invasion, and metastatic colonization. It also allows for localized therapeutic delivery and real-time monitoring of treatment response. Histologically, orthotopically implanted RKO tumors replicate the poorly differentiated phenotype observed in human tumors, supporting their relevance in translational research. Given RKO’s distinct molecular profile, this model facilitates exploration of how mismatch repair deficiency and epigenetic modifications shape tumor biology and therapeutic response. Future studies incorporating humanized immune systems and refined implantation techniques may further enhance the clinical applicability of RKO orthotopic xenografts, contributing to the development of more effective and personalized treatment strategies.
Enhanced Antitumor Activity of SOMCL-19-133 in RKO Colon Cancer Models
A study by Zhou et al., published in Neoplasia journal, describes the development of SOMCL-19-133, a selective and orally bioavailable inhibitor of the NEDD8-activating enzyme (NAE). The compound demonstrates potent enzymatic inhibition (IC₅₀ = 0.36 nM) and exceptional selectivity over the closely related ubiquitin-activating enzyme. In both in vitro and in vivo experiments, SOMCL-19-133 induced accumulation of CRL substrates such as Cdt1 and p21, triggered DNA damage signaling via γH2AX, and led to apoptosis in human colon cancer cells including RKO. In RKO specifically, SOMCL-19-133 showed enhanced effects compared to MLN4924, with a lower IC₅₀ (40.23 ± 5.12 nM versus 150.5 ± 26.38 nM) and greater induction of cell cycle arrest and polyploidy. In a xenograft model using RKO tumors, oral administration of SOMCL-19-133 significantly reduced tumor volume in a dose-dependent manner, yielding a T/C value of 0.03 at 10 mg/kg, which was more effective than MLN4924. The methods employed included enzyme inhibition assays, UBL-thioester loading, flow cytometry, western blotting, and xenograft models, with careful control and replication. RKO and HCT-116 cells were used to confirm the compound’s effects across genetically distinct colorectal lines.
Targeting Geranylgeranylation Disrupts RKO Cell Growth
The article authored by Wang X, et al. and published in Molecular Cancer journal investigates the effects of lipophilic bisphosphonate BPH1222, a geranylgeranyl diphosphate synthase (GGDPS) inhibitor, on colorectal cancer cell lines, with particular emphasis on RKO. The central hypothesis posits that BPH1222 suppresses cell proliferation through inhibition of protein geranylgeranylation, ultimately disrupting small GTPase activity. Key findings show that BPH1222 significantly inhibits viability in multiple colon cancer cell lines, with RKO being among the most sensitive. Specifically, BPH1222 reduced RKO cell viability in a dose-dependent manner and caused marked downregulation of Rac1 and Rab6A protein levels, which are crucial for actin cytoskeleton organization and vesicular trafficking. Additionally, flow cytometry revealed a prominent increase in G1 phase arrest in RKO cells, and western blot analyses indicated decreased levels of phosphorylated ERK1/2 and cyclin D1. The authors further showed that BPH1222 suppressed anchorage-independent growth of RKO cells and that co-treatment with GGOH partially rescued cell viability, confirming the specificity of the drug’s mechanism. These findings strongly support the paper’s thesis that GGDPS inhibition via BPH1222 impairs post-translational modification of small GTPases, which are integral to cancer cell survival and proliferation. A notable pattern is the selective sensitivity of RKO cells to BPH1222, possibly due to their specific dependency on geranylgeranylation-dependent pathways. The experimental methods were comprehensive, utilizing multiple assays including MTT, flow cytometry, soft agar colony formation, and western blotting.
RKO Cell Sensitivity to Dual Deubiquitinase Inhibition
The RKO colorectal cancer cell line was evaluated to determine its response to a novel dual inhibitor targeting USP28 and USP25, two deubiquitinases implicated in oncogenic protein stabilization. The compound induced a marked reduction in RKO cell viability, with a low nanomolar IC50, indicating high potency. Treatment led to the depletion of c-Myc, a key oncogenic driver, as well as reduced levels of Notch1 and c-Jun proteins. This downregulation correlated with increased markers of apoptosis, including cleaved PARP and caspase-3, as well as elevated γ-H2AX, suggesting that DNA damage was induced as part of the compound’s cytotoxic mechanism. The compound also impaired clonogenic potential and induced G1 cell cycle arrest, further supporting its inhibitory effect on tumor cell proliferation. A notable pattern was the compound’s selectivity against tumors with elevated c-Myc and Notch1 activity, positioning RKO as a highly responsive model due to its inherent molecular characteristics. These findings strongly support the hypothesis that USP28/USP25 inhibition destabilizes oncogenic networks in susceptible cancer types. The methods employed included standard in vitro assays such as cell viability screening, immunoblotting, and flow cytometry, all of which were appropriate for delineating molecular and phenotypic outcomes. However, the absence of in vivo validation or extended time-course analyses presents a limitation in assessing long-term efficacy and systemic tolerability. The findings affirm RKO as a valuable preclinical model for mechanistic studies of oncogene-driven tumor suppression via ubiquitin pathway disruption.
Synergistic Inhibition of Colon Tumors via Akt and EGFR Co-Blockade
The experimental findings demonstrate that the Akt inhibitor ISC-4 synergizes with cetuximab to produce a potent antitumor response in human colon cancer cells, particularly those with wild-type KRAS such as RKO. Initial screens identified ISC-4 as having modest single-agent efficacy in colon cancer models. However, its potency significantly increased when combined with cetuximab, specifically in wild-type KRAS cells. This synergy was characterized by enhanced apoptosis, increased sub-G1 cell population, and reduced phospho-Akt levels. In vitro, this combinatorial treatment induced early and sustained cytotoxic responses, including caspase-3 activation and DNA fragmentation. Notably, the synergistic effect persisted in RKO clones with acquired resistance to 5-FU, emphasizing the clinical relevance of this approach for refractory disease. In vivo studies in RKO xenograft models confirmed the combination’s efficacy in reducing tumor growth without observed toxicity, as measured by stable body weights and unaltered serum chemistry.
RKO Colon Cancer CRC Orthotopic And Subcutaneous Xenograft Model: Download ![]()
Basic study design
- Each athymic BALB/c (nu/nu) mouse (10 weeks old) receives a single s.c. injection into the hind leg. The cell inoculation contains 1 x 106 cells (in 100 µL injection volume) of the Matrigel+RKO cell suspension.
- The mice are observed until tumors are established. Tumors are expected to reach 50-150 mm3 before dosing begins. Animals are randomized into treatment groups.
- Tumors (daily) and body weights (tri-weekly) are recorded.
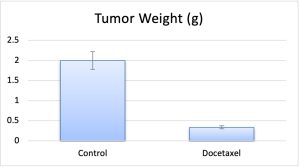
- The study ends as tumors reach maximum tumor size limits (2,000 cu. millimeters). Tumors are resected from the animal and weighed. A standard necropsy is performed; tissues are collected following the customer’s request.
- Tumors and/or tissues are frozen in liquid nitrogen, prepared for histology in 10% NBF or stabilized in RNAlater reagent.
- Animals are housed in a pathogen-free facility following the Guide for Care and Use of Laboratory Animals, along with regulations of the Institutional Animal Care and Use Committee (IACUC).
Get Instant Quote for
RKO Xenograft Model
Xenograft animal models are used to assess the effectiveness of drugs against specific types of cancer. New medicines are tested on staged tumor growths that have been engrafted via subcutaneous or orthotopic inoculation in an immunocompromised mouse or rat model. All clinically approved anti-cancer agents have been evaluated with conventional preclinical in vivo models. Xenograft studies can be highly complex, starting with the selection of the appropriate animal model, choice of tumorigenic cell line, administration method, dosing, analysis of tumor growth rates and tumor analysis (histology, mRNA and protein expression levels).
Following options are available for the RKO xenograft model:
- RKO Tumor Growth Delay (TGD; latency)
- RKO Tumor Growth Inhibition (TGI)
- Dosing frequency and duration of dose administration
- Dosing route
- RKO tumor immunohistochemistry
- Blood chemistry analysis
- Toxicity and survival (optional: performing a broad health observation program)
- Gross necropsies and histopathology
- Positive control group employing cisplatin, at a dosage of 25-30 mg/kg
- Lipid distribution and metabolic assays
- Imaging studies: Fluorescence-based whole body imaging, MRI
