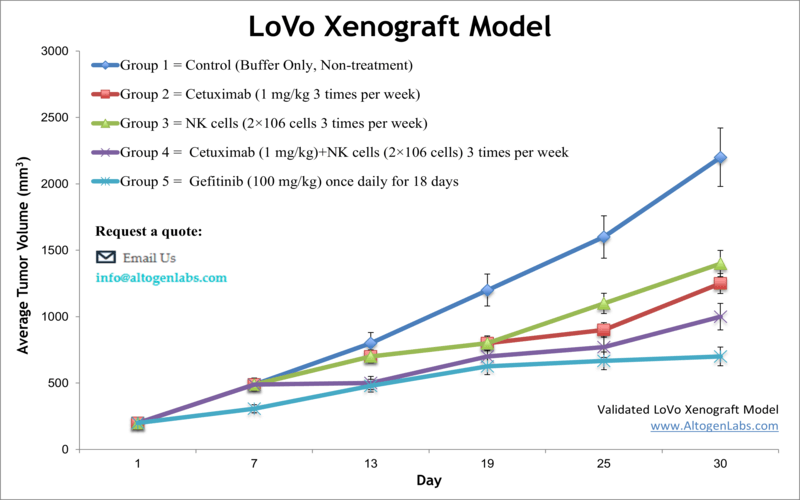
LoVo xenograft model
Colorectal carcinoma is the third most frequently diagnosed malignancy in the United States, accounting for roughly 50,000 deaths annually, as indicated by the American Cancer Society (ACS). The LoVo xenograft model aids in the discovery of innovative anti-cancer drugs, by accurately reflecting molecular characteristics of human tumors. The LoVo epithelial cell line was isolated from a metastatic tumor nodule fragment in the left supraclavicular region of a 56-year-old Caucasian male patient with colorectal adenocarcinoma by Drewinko et al. in 1976. The LoVo cell line is a well-differentiated cell line that presents most of the properties of intestinal cells, including the formation of acinar structures, microvilli, a glycocalyx, desmosomes, adherens and tight junctions, as per a 2003 study in Oncogene. LoVo has proven to be a potent tool to study loss of epithelioid organization. LoVo cells are negative for expression of colon antigen 3 and colon-specific antigen-p (CSAp-). Also, these cells express N-ras, H-ras, Myb, c-myc, K-ras, sis and fos oncogenes. A 2012 study by Zou et al. used the LoVo cell line and xenograft to demonstrate the anticancer properties of Beta-asarone, a component of the common traditional Chinese medicine Acorus calamu. Results presented the novel finding that β-aserone induces apoptosis and reduces tumor growth in colon cancer via mitochondria and caspase pathways. The study by Piotrowska et al. (2013) used the LoVo cell model to compare the cytotoxicity of known anticancer agent DMU-212; results indicated that induction of apoptosis is due to upregulation of pro-apoptotic transcripts (Bik, Bak1, Bad, Bok, p52, Apaf1, Noxa) and downregulation of anti-apoptotic factors (Bag1, Bcl-xL, Bcl-2) and that cytochrome p450 catalyzes DMU-212 biotransformation that is critical to anticancer effects. Finally, the 2016 Oncology Letters study (Chen et al.) used the LoVo xenograft model in testing efficacy of cetuximab to mediate activity of antibody dependent cell mediated cytotoxicity (ADCC) via natural killer (NK) cells by increasing specificity on epidermal growth factor receptor (EGFR) positive colorectal cancer cells; results showed cetuximab successfully increased the intensity of ADCC activity which could provide a potential immunotherapy for CRC patients exhibiting metastasis and EGFR expression. The LoVo cell line is used to create the CDX (Cell Line Derived Xenograft) LoVo xenograft mouse model. The LoVo xenograft model is a preclinical system that allows an understanding of the mechanism of action (i.e. quantitation of gene expression changes, pathway analysis, biomarkers) following treatment with VEGR-TKIs (e.g. cediranib).
LoVo Colon Cancer Subcutaneous And Metastatic Xenograft Model ![]()
Download Altogen Labs LoVo Xenograft Model PowerPoint Presentation: ![]()
LoVo Cell Line
The LoVo cell line, derived from a metastatic colorectal adenocarcinoma lesion, is widely utilized in preclinical oncology research as a representative model of advanced-stage colorectal cancer. Characterized by a KRAS G13D mutation, wild-type BRAF, and microsatellite instability-high (MSI-H) status, LoVo cells offer a clinically relevant platform for investigating mechanisms of drug resistance, metastasis, and immune modulation. Studies have demonstrated that LoVo cells exhibit upregulation of thymidylate synthase and ATP-binding cassette transporters in response to 5-fluorouracil and other chemotherapeutic agents, underscoring their utility in resistance profiling. Furthermore, LoVo cells display features of epithelial-mesenchymal transition following TGF-β induction, with increased expression of transcriptional repressors such as Snail and ZEB1. While their aggressive growth and angiogenic potential have been confirmed in xenograft models, LoVo tumors show limited response to anti-angiogenic therapies, suggesting a complex, VEGF-independent vascularization mechanism. Although their MSI-H phenotype implies potential immunogenicity, functional studies examining immune evasion pathways, particularly PD-L1 expression and tumor-immune interactions, remain underdeveloped.
Subcutaneous LoVo Xenografts in Colorectal Cancer Research
Subcutaneous xenograft transplantation is a widely adopted in vivo model for studying tumor growth, therapeutic efficacy, and molecular signaling in colorectal cancer. By injecting human cancer cells into the subcutaneous tissue of immunocompromised mice, this model offers a reproducible and accessible system for longitudinal tumor monitoring. The LoVo colorectal adenocarcinoma cell line, known for its KRAS G13D mutation and microsatellite instability-high status, has been extensively utilized in such xenograft studies. LoVo-derived tumors reliably form subcutaneous masses that retain key molecular characteristics of the parental line, including upregulation of thymidylate synthase and efflux transporters, which are associated with resistance to chemotherapeutic agents such as 5-fluorouracil and oxaliplatin. Functional studies involving gene silencing or overexpression in LoVo cells have demonstrated significant effects on tumor growth in vivo, underscoring the model’s value in validating therapeutic targets and elucidating mechanisms of drug resistance.
While subcutaneous models offer practical advantages in terms of tumor accessibility and volume measurement, they do not fully replicate the tumor-stroma interactions or the immunologic milieu of the colorectal tumor microenvironment. The use of immunocompromised hosts limits investigation of immune checkpoint inhibition or other immunotherapies, and the ectopic tumor site lacks the physiological cues present in the gastrointestinal tract. Nevertheless, LoVo xenografts remain a cornerstone of preclinical colorectal cancer research, particularly for evaluating drug responses and conducting mechanistic studies under controlled conditions. Advances such as the use of humanized mouse models and co-injection with stromal components are expanding the relevance of subcutaneous xenografts, allowing researchers to bridge the gap between in vitro findings and clinical application. Incorporating LoVo subcutaneous xenografts into a multifaceted experimental approach will continue to provide valuable insights into colorectal cancer pathobiology and treatment resistance.
LoVo-Based In Vivo Systems for Colorectal Cancer Metastasis
Metastatic xenograft transplantation is a critical component of preclinical colorectal cancer research, providing insight into the mechanisms governing tumor dissemination and the evaluation of therapeutic strategies targeting metastatic disease. The LoVo colorectal adenocarcinoma cell line, with its KRAS G13D mutation and microsatellite instability-high status, has been extensively used to establish metastatic models due to its aggressive phenotype and reproducible metastatic behavior in vivo. Experimental metastasis models typically involve intravenous injection of LoVo cells into immunodeficient mice, enabling the study of hematogenous spread and colonization of distant organs, particularly the lungs. While metastatic LoVo models offer considerable advantages in mechanistic and therapeutic research, certain limitations must be acknowledged. The requirement for immunodeficient host mice precludes investigation of immune-mediated mechanisms of metastasis, and variability in metastatic colonization efficiency poses challenges for reproducibility. Furthermore, the experimental nature of intravenous injection bypasses the early stages of the metastatic cascade, such as local invasion and intravasation, limiting its physiological fidelity. Nonetheless, metastatic xenograft models using LoVo cells remain indispensable for delineating molecular drivers of metastasis, evaluating drug efficacy in advanced disease stages, and identifying potential biomarkers of metastatic progression. Advancements in imaging technologies and humanized mouse platforms may further enhance the translational applicability of these models and contribute to the development of more effective treatments for metastatic colorectal cancer.
Enhanced Antitumor Activity of HCPT Nanoparticles in LoVo Xenografts
This study by Wang and Li, published by BMC Biotechnology journal, investigates the therapeutic efficacy of hydroxycamptothecin (HCPT) encapsulated in PEG-PBLG nanoparticles, with a specific focus on LoVo colon cancer xenografts. The authors report that the HCPT-loaded nanoparticles possess a spherical core-shell structure, featuring a 200 nm hydrophobic core and a 30 nm hydrophilic shell. This architecture facilitates controlled drug delivery and reduces uptake by the reticuloendothelial system. In vitro release studies revealed a biphasic pattern with an initial rapid release followed by sustained release. In vivo pharmacokinetic analyses demonstrated that encapsulation extended the plasma half-life of HCPT from 4.5 hours to 10.1 hours, reduced the peak plasma concentration, and increased the apparent volume of distribution from 7.3 liters to 20.0 liters. These changes reflect improved circulation time and enhanced tissue delivery. In LoVo xenograft models, the HCPT-loaded nanoparticles achieved a tumor inhibition rate of 83.8 percent, compared to 70.0 percent with free HCPT. Tumor doubling time increased, and no additional systemic toxicity was observed, suggesting a favorable therapeutic profile. The nanoparticle formulation helps preserve the lactone form of HCPT, which is essential for its activity against topoisomerase I. While the results are promising, the study has some limitations. It utilizes only one tumor model and a relatively small sample size, which may affect the broader applicability of the conclusions. Furthermore, the lack of detailed mechanistic exploration of cellular uptake or biodistribution limits understanding of tissue-specific effects. The study also does not address immune-related responses, given the use of immunodeficient mice. Despite these limitations, the work highlights the significant potential of PEG-PBLG nanoparticles for improving chemotherapy efficacy.
Anti-Angiogenic and Immunomodulatory Effects of Rg3 in LoVo Cells
Ginsenoside Rg3, a pharmacologically active compound derived from ginseng, exerts multiple antitumor effects in colorectal cancer cells, particularly in the LoVo cell line. In vitro, treatment with Rg3 reduces LoVo cell viability and migration in a dose- and time-dependent manner. These functional changes are associated with decreased expression of cancer stem cell markers including CD24, CD44, and EpCAM. Flow cytometry and real-time PCR confirm reduced protein and mRNA levels of these markers, suggesting diminished self-renewal and tumor-initiating capacity. Clonogenic assays show that Rg3 impairs the colony-forming ability of LoVo cells, further supporting a loss of stem-like properties. In vivo, LoVo-derived orthotopic xenografts exhibit significant tumor regression after Rg3 administration, accompanied by lower expression of proliferation and stemness indicators such as Ki-67, CD24, CD44, and EpCAM in tumor tissue. Ginsenoside Rg3 also modifies the tumor microenvironment by reducing angiogenesis and enhancing therapeutic sensitivity. Treated LoVo tumors exhibit decreased microvessel density, and analysis of angiogenesis-related gene expression reveals broad downregulation of pro-angiogenic factors including ANGPT1, KDR, MMP1, IL8, and PGF. These genes are involved in endothelial proliferation, matrix remodeling, and inflammatory signaling, which collectively sustain tumor vascularization. Rg3 enhances the efficacy of conventional chemotherapeutic agents such as 5-fluorouracil and oxaliplatin, leading to greater tumor reduction than chemotherapy alone. Additionally, Rg3 lowers the expression of immune checkpoint molecules B7-H1 and B7-H3, which are associated with poor clinical outcomes and immune evasion in colorectal cancer. While further validation in immunocompetent systems is needed, these results suggest that Rg3 contributes to antitumor immunity. Together, these findings highlight the relevance of LoVo as a model for studying cancer stem cell dynamics, angiogenesis, and immune modulation, and support the potential of Rg3 as a multi-target therapeutic candidate in colorectal cancer.
Survivin Suppression Triggers Apoptosis in LoVo Colon Cancer Cells
Survivin, a key member of the inhibitor of apoptosis protein family, is frequently overexpressed in colorectal cancer and plays a crucial role in preventing programmed cell death. In LoVo colorectal cancer cells, suppression of survivin has been shown to significantly reduce tumor cell viability and promote apoptosis. This process is closely linked to the mitochondrial pathway of apoptosis, where decreased survivin levels are associated with a reduction in mitochondrial membrane potential and the subsequent release of cytochrome C into the cytosol. Cytochrome C activates caspase-9, which in turn activates downstream effector caspases such as caspase-3 and caspase-7, culminating in cleavage of PARP and execution of the apoptotic program. Experimental evidence shows that small-molecule inhibitors can effectively target survivin expression, leading to marked changes in the expression of apoptosis-related proteins in LoVo cells. These include decreased levels of Bcl-2, an anti-apoptotic protein, and increased levels of cleaved caspase-3, -7, and -9, as well as cleaved PARP and cytochrome C. Such molecular changes correlate with reduced tumor growth in vivo and increased apoptosis in vitro. Importantly, combining survivin inhibitors with other agents can enhance these effects, suggesting that co-targeting survivin and related pathways may improve therapeutic efficacy. The LoVo model is particularly well-suited for studying mitochondrial-mediated apoptosis and serves as a valuable platform for investigating targeted therapies in colorectal cancer. These findings underscore the therapeutic potential of modulating apoptosis regulators to suppress tumor growth and highlight survivin as a promising molecular target for intervention.
LGR5 Loss Drives Resistance in LoVo Cells via MET-STAT3 Activation
In LoVo colorectal cancer cells, loss of LGR5 through chemotherapy, gene ablation, or LGR5-targeted therapies induces a shift to a more drug-resistant phenotype. This shift is marked by enhanced clonogenic survival, even in the presence of cytotoxic agents such as irinotecan. Mechanistically, LGR5 loss activates the MET-STAT3 signaling axis, which in turn drives transcription of survival genes including Cyclin D1 and Bcl-xL. LoVo cells with reduced LGR5 levels show elevated phosphorylation of MET and STAT3, a relationship supported by both gene expression and protein analyses. Inhibition of either MET or STAT3 suppresses this signaling, while constitutively active STAT3 promotes MET activity and downregulates LGR5, indicating a positive feedback loop. Importantly, reintroduction of LGR5 reverses this effect, suppressing MET-STAT3 signaling and restoring drug sensitivity. These findings highlight a reciprocal regulatory mechanism where LGR5 loss fuels STAT3-driven resistance, positioning MET and STAT3 as therapeutic targets in LGR5-negative colorectal cancer populations. Pharmacologic inhibition of MET and STAT3 enhances the response to irinotecan and LGR5-directed therapies in LoVo cells, especially those lacking LGR5. Combination treatments result in synergistic suppression of tumor viability in vitro and significantly reduce tumor burden in xenograft models, accompanied by improved survival outcomes. Patient-derived tumor organoids support the utility of this strategy in clinically relevant settings. The LoVo model serves as a robust platform to study the dynamics of cancer cell plasticity and therapeutic resistance driven by LGR5 status. These findings underscore the importance of targeting both LGR5-positive and LGR5-negative tumor subpopulations to overcome treatment failure. Further research is needed to elucidate the upstream regulators of MET processing in the absence of LGR5 and to explore the broader applicability of this combination approach in heterogeneous colorectal cancer settings.
RICTOR Regulates TELO2 Stability and Oncogenic Activity in LoVo Cells
The LoVo colorectal cancer cell line expresses high levels of TELO2, a telomere maintenance protein that plays a pivotal role in mTOR complex stabilization and signaling. In LoVo cells, TELO2 acts as a promoter of tumorigenic behavior, including proliferation, migration, and invasion. When TELO2 expression is silenced, LoVo cells show a marked reduction in anchorage-independent growth, decreased cell motility, and suppressed invasion capacity. These phenotypes coincide with arrest in the G1 phase of the cell cycle. TELO2 protein is expressed in both the cytoplasm and nucleus and correlates positively with RICTOR, a component of mTORC2. Co-immunoprecipitation and immunohistochemical analyses confirm a strong interaction between TELO2 and RICTOR in both cultured cells and colorectal cancer tissue samples. TELO2 expression levels are higher in early-stage tumors and correlate with patient age but show an inverse relationship with lymph node metastasis and advanced TNM stage. Mechanistically, TELO2 promotes tumor progression by engaging the mTORC2 pathway in a serum-dependent manner. In LoVo cells cultured with serum, TELO2 enhances phosphorylation of Akt at Ser473, a key mTORC2 target that drives cell survival and proliferation. Knockdown of RICTOR disrupts this signaling, impairs the tumorigenic effects of TELO2, and causes cell cycle arrest. Under serum-deprived conditions, however, TELO2 is destabilized via RICTOR-mediated ubiquitination, independent of mTOR, suggesting a dual regulatory role for RICTOR depending on the cellular environment. The positive feedback between TELO2 and RICTOR is absent at the mRNA level, indicating that regulation occurs post-translationally. These findings highlight a context-dependent oncogenic function of TELO2 in LoVo cells and reveal that serum availability determines whether TELO2 promotes tumor growth or is targeted for degradation.
LoVo Colon Cancer Subcutaneous And Metastatic Xenograft Model ![]()
Basic study design
- Cells are collected by trypsinizing the cells. Viability is then determined with trypan blue, requiring a minimum of 98% viability to initiate the study. Suspension cell concentrations are adjusted to the appropriate density needed for inoculation.
- After dilution to the correct concentration, one million LoVo cells with 50% Matrigel (100 µL vol) is injected subcutaneously (s.c.) in the flank of each mouse (athymic BALB/C, 10 to 12 weeks).
- Calipers (digital) are used for measuring tumor progression. Randomization of mice into the appropriate treatment cohorts marks the start of the study. Test compounds are injected as instructed by the client.
- Tumor measurements and mouse weights are documented (client specified days of measurement).
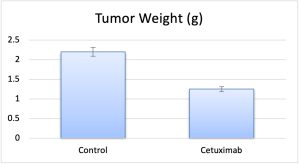
- At end of study, the animals are euthanized humanely and a necropsy is performed to remove tumors. Tumor weight is recorded and is digitally imaged.
- Tissues are collected by standard gross necropsy. Tissues are snap frozen or submersed in RNAlater or nucleic acids isolated.
Get Instant Quote for
LoVo Xenograft Model
Animal handling and maintenance at the Altogen Labs facilities are IACUC-regulated and GLP-compliant. Following acclimatization to the vivarium environment, mice are sorted according to body mass. The animals are examined daily for tumor appearance and clinical signs. We provide detailed experimental procedures, health reports and data (all-inclusive report is provided to the client that includes methods, results, discussion and raw data along with statistical analysis). Additional services available include collection of tissue, histology, isolation of total protein or RNA and analysis of gene expression.
Following options are available for the LoVo xenograft model:
- LoVo Tumor Growth Delay (TGD; latency)
- LoVo Tumor Growth Inhibition (TGI)
- Dosing frequency and duration of dose administration
- Dosing route (intravenous, continuous infusion, intraperitoneal, intratumoral, oral gavage, subcutaneous)
- LoVo tumor immunohistochemistry
- Blood chemistry analysis
- Toxicity and survival (optional: performing a broad health observation program)
- Gross necropsies and histopathology
- Positive control group employing dox or cyclophosphamide, at a dosages of 20-50 mg/kg
- Lipid distribution and metabolic assays
- Imaging studies: Fluorescence-based whole body imaging, MRI
