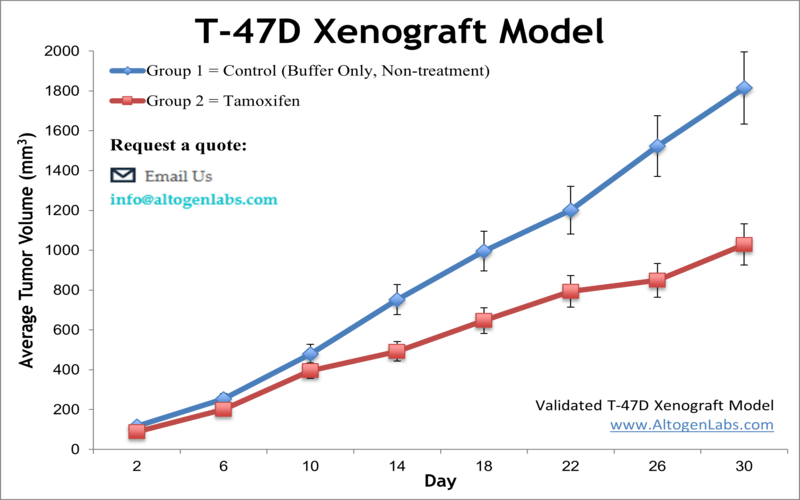
T-47D xenograft model
The T47D xenograft model is an estrogen receptor-positive (ER+), progesterone receptor-positive (PR+), HER2-negative breast cancer model. It is hormone-responsive, making it relevant for studying endocrine therapy resistance. Key mutations include TP53 L194F (loss-of-function, impairing DNA damage response) and PIK3CA H1047R (activating PI3K/AKT signaling, driving proliferation and therapy resistance). Additional alterations in RB1 and CDKN2A affect cell cycle regulation. This model is crucial for evaluating endocrine therapies and targeted treatments for ER+ breast cancer patients. T47D is a human breast cancer cell line that was established from a patient with an infiltrating ductal carcinoma, which is the most common type of breast cancer. The T-47D hypotriploid epithelial cell line is derived from ductal carcinoma of the breast of a 54-year-old female patient and is a suitable transfection host. The T-47D cell line has been routinely utilized in breast cancer research. Examples include the 2017 Nature study by Riggio et al. that examined the mechanisms behind AKT1 and AKT2 isoforms in promoting breast cancer progression; results showed in vitro AKT1 promoted proliferation and growth and inhibited cell migration while AKT2 promoted cell migration and invasion. This study highlighted the importance of differentiating between isoforms and concluded that AKT2 upregulation would make a reasonable target due to its link to metastasis which in turn contributes to poor prognosis. A 2016 study by Puchalapalli et al. used the T47D model for its estrogen receptor positive (ER+) phenotype in order to determine the optimal mouse model for studying spontaneous metastasis and concluded that NOD scid gamma (NSG) mice are a better model compared to nude mice as NSG mice demonstrate more severe tumor growth, metastases, and metastatic burden. A 2006 Cancer Research article (Hartman et al.) used the T47D xenograft model to test the proliferative effects of estrogen receptor β (ER-β), which has reported antiproliferative effects as opposed to ER-α which has estrogen-mediated proliferative effects on normal and tumor breast cells. Results demonstrated that the introduction of ER-β into the xenograft model reduced tumor growth, intramural blood vessels. VEGF expression, and platelet-derived growth factor β (PDGFβ), suggesting that the mechanism of action of growth inhibition is via angiogenesis inhibition. The T-47D cell line is used to create xenograft mouse model. The T-47D xenograft model is estrogen receptor positive and progesterone receptor positive and lends itself to anti-tumor studies taking into consideration receptor status (e.g. quinoline) and angiogenesis (e.g. ERβ replacement therapy).
T47D Subcutaneous And Orthotopic Xenograft Model: Download ![]()
Download Altogen Labs T47D Xenograft Model PowerPoint Presentation: ![]()
Orthotopic T-47D xenograft model
The orthotopic T-47D xenograft model is a widely utilized preclinical system for studying estrogen receptor-positive (ER+) breast cancer in a biologically relevant microenvironment. In this model, T-47D cells are implanted directly into the mammary fat pad of immunocompromised mice, allowing for tumor growth in a site that closely mimics the natural tumor niche. Unlike subcutaneous xenografts, orthotopic implantation preserves key tumor-stroma interactions, facilitating more accurate assessments of tumor progression, invasion, and response to endocrine therapies. Since T-47D cells require estrogen for optimal growth in vivo, estrogen supplementation is typically provided to maintain tumor viability and replicate hormone-driven breast cancer conditions. This model is particularly valuable for evaluating selective estrogen receptor modulators (SERMs), aromatase inhibitors, and combination therapies targeting hormone receptor pathways. Additionally, the orthotopic setting enables the study of metastasis, as tumors can disseminate to secondary sites such as lymph nodes and distant organs. By integrating molecular and histological analyses, the orthotopic T-47D model provides critical insights into tumor biology and therapeutic resistance, supporting the development of more effective breast cancer treatments.
Chromosomal Instability in T-47D: A Triploid Karyotype with Structural Aberrations
T-47D breast cancer cells exhibit a highly abnormal karyotype characterized by a near-triploid (3n) chromosome number, ranging from 57 to 66 chromosomes. These cells display extensive numerical and structural chromosomal alterations, including deletions, duplications, and translocations. A notable feature of T-47D cells is the frequent loss of chromosomes 1, 2, 4, and 6, as well as the presence of derivative chromosomes resulting from complex rearrangements. Among the most recurrent structural aberrations are translocations involving chromosomes X, 6, 8, 10, and 20, highlighting significant genomic instability. Polyploidy is observed in a subset of T-47D cells, suggesting ongoing chromosomal instability that may contribute to tumor heterogeneity. Comparative cytogenetic analysis reveals that T-47D shares some chromosomal abnormalities with BT474 cells but remains distinct from MCF7, another luminal A breast cancer model. These chromosomal alterations could have implications for gene expression, therapeutic response, and disease progression in ER+/HER2- breast cancer. Understanding the karyotypic complexity of T-47D cells is crucial for their use in breast cancer research and drug development.
T-47D model used in breast cancer research studies
Calycosin, a natural isoflavone, has shown promising anti-cancer effects by inhibiting the migration and invasion of T-47D breast cancer cells. It achieves this by downregulating BATF, a transcription factor that promotes tumor progression, leading to the suppression of TGFβ1, a key driver of epithelial-mesenchymal transition (EMT). EMT is a crucial process in cancer metastasis, where epithelial cells lose their cell-to-cell adhesion properties and gain migratory and invasive capabilities. In T-47D cells, calycosin treatment resulted in increased expression of E-cadherin (an epithelial marker) and decreased levels of mesenchymal markers like N-cadherin, Vimentin, and matrix metalloproteinases (MMP-2 and MMP-9). Functional assays confirmed that calycosin significantly reduced tumor cell motility and invasive potential. Additionally, in vivo studies demonstrated that calycosin effectively suppressed tumor growth derived from T-47D cells. These findings suggest that calycosin is a promising therapeutic candidate for breast cancer, particularly for targeting metastasis through the BATF/TGFβ1 axis.
A study conducted by Wang S, et al., published by Cell Death and Disease journal investigates the effects of DHW-208, a novel 4-aminoquinazoline derivative, on breast cancer cells, with a particular focus on the T-47D cell line. The research highlights that DHW-208 effectively inhibits the proliferation, migration, and invasion of T-47D cells by targeting the PI3K/AKT/mTOR signaling pathway, a crucial regulator of cancer cell survival. Through in vitro experiments, it was demonstrated that DHW-208 induces apoptosis via the mitochondrial pathway and promotes G0/G1 cell cycle arrest in T-47D cells, thereby restricting tumor growth. The compound also exhibited significant anti-tumor effects in an in vivo xenograft model using T-47D cells, further confirming its potential as a therapeutic candidate. Western blot analysis revealed that DHW-208 suppresses the phosphorylation of key proteins in the PI3K/AKT/mTOR axis, indicating its role as a dual PI3K/mTOR inhibitor. Furthermore, DHW-208 outperformed BEZ235, a known PI3K/mTOR inhibitor, in reducing tumor growth with lower toxicity. The study provides strong evidence that DHW-208 is a promising drug candidate for breast cancer therapy, particularly for PI3K/AKT/mTOR-driven cancers like T-47D.
Another study conducted by Wang J, et al., published by The Anatomical Record journal investigates the role of RHAMM (Receptor for Hyaluronan-Mediated Motility) in regulating cell migration in luminal A breast cancer, focusing on T-47D cells. Contrary to its well-established role in promoting motility in many cancers, RHAMM was found to inhibit migration in T-47D cells. Knockdown of RHAMM in T-47D cells led to an increase in migration and epithelial-to-mesenchymal transition (EMT), marked by reduced E-cadherin expression and elevated Snail levels. Mechanistically, RHAMM deficiency stabilized Snail by enhancing AKT-mediated phosphorylation of GSK3β, leading to decreased Snail degradation and increased pro-migratory signaling. Functional assays, including wound healing and transwell invasion experiments, confirmed that loss of RHAMM significantly enhanced T-47D cell migration. Additionally, in vivo models demonstrated that RHAMM knockdown increased lung metastasis of T-47D cells in NOD/SCID mice. These findings reveal a previously unrecognized function of RHAMM as a migration suppressor in luminal A breast cancer and suggest that targeting RHAMM-related pathways could influence metastasis in T-47D-driven tumors.
Basic study design
- T-47D cells are specifically maintained under exponential growth for in vivo studies. The cells are collected via trypsinization and viability and cell counts are determined. Concentration of the cell suspension is diluted to the working injection volume.
- Mice (NOD/SCID, 7-8 weeks old) receive a subq injection in the hind leg that contains one million cells (Matrigel plus T-47D cells; volume 150 µl).
- Injections are monitored until tumor establishment. Digital calipers are used to measure tumors; the in-life part of the study begins as tumors reach an average size of 150-200 mm3, at which point the animals are randomized to form study groups.
- The dosing schedule is followed for administration of the compound of interest.
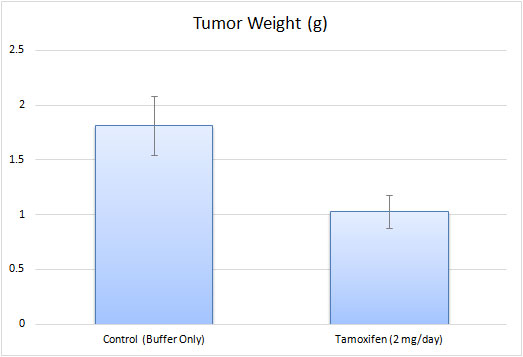
- Tumor measurements occur daily, along with body weights being recorded 2-3 times a week. At the end of study, necropsies are performed and samples are collected according to the tissue collection sheet. All tumors are resected from the mice and weighed. Tumor and tissue samples can be frozen in liquid nitrogen, stored in 10% NBF for histology, or isolated for genetic analysis.
Get Instant Quote for
T-47D Xenograft Model
Animal handling and maintenance at the Altogen Labs facility is IACUC-regulated and GLP-compliant. Following acclimation to the vivarium environment, mice are sorted according to body mass. The animals are examined daily for tumor appearance and clinical signs. We provide detailed experimental procedures, health reports and data (all-inclusive report is provided to the client that includes methods, results, discussion and raw data along with statistical analysis). Additional services available include collection of tissue, histology, isolation of total protein or RNA and analysis of gene expression.
Xenograft animal models are used to assess the effectiveness of drugs against specific types of cancer. New medicines are tested on staged tumor growths that have been engrafted via subcutaneous or orthotopic inoculation in an immunocompromised mouse or rat model. All clinically approved anti-cancer agents have been evaluated with conventional preclinical in vivo models. Xenograft studies can be highly complex, starting with the selection of the appropriate animal model, choice of tumorigenic cell line, administration method, dosing, analysis of tumor growth rates and tumor analysis (histology, mRNA and protein expression levels).
Altogen Labs provides an array of laboratory services using over 90 standard Cell Line Derived Xenograft (CDX) models and over 30 PDX models. Researchers investigating the role of specific proteins or gene products in regulating tumor growth can benefit from development of protein overexpression (genetically engineered to ectopically express proteins, tumor suppressors, or oncogenes) and RNAi cell lines with long term gene silencing. Altogen Labs provides quantitative gene expression analysis of mRNA expression (RT-PCR) and protein expression analysis using the WES system (ProteinSimple).
Following options are available for the T-47D xenograft model:
- T-47D Tumor Growth Delay (TGD; latency)
- T-47D Tumor Growth Inhibition (TGI)
- Dosing frequency and duration of dose administration
- Dosing route (intravenous, intratracheal, continuous infusion, intraperitoneal, intratumoral, oral gavage, topical, intramuscular, subcutaneous, intranasal, using cutting-edge micro-injection techniques and pump-controlled IV injection)
- T-47D tumor immunohistochemistry
- Alternative cell engraftment sites (orthotopic transplantation, tail vein injection and left ventricular injection for metastasis studies, injection into the mammary fat pad, intraperitoneal injection)
- Blood chemistry analysis
- Toxicity and survival (optional: performing a broad health observation program)
- Gross necropsies and histopathology
- Positive control group employing cyclophosphamide, at a dosage of 50 mg/kg administered by intramuscular injection to the control group daily for the study duration
- Lipid distribution and metabolic assays
- Imaging studies: Fluorescence-based whole body imaging
