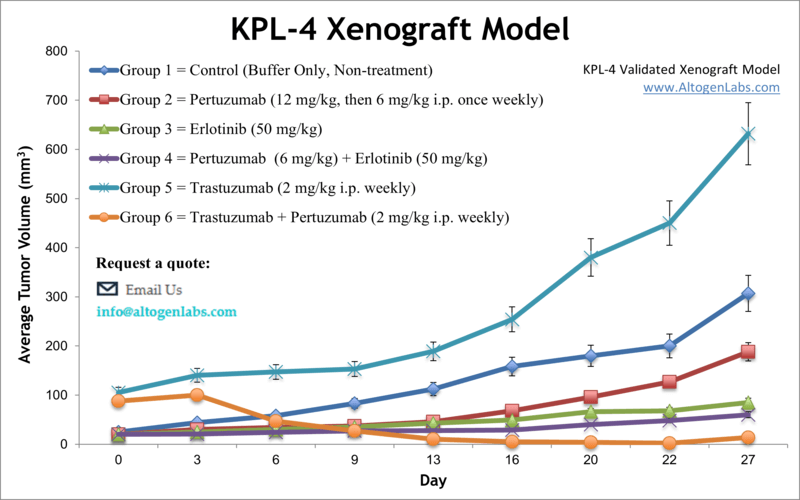
KPL-4 xenograft model
KPL-4 is a human breast cancer cell line that was originally derived from a breast cancer patient with metastasis to the axillary lymph node. These cells have been widely used as a model system for studying various aspects of breast cancer biology, including the molecular mechanisms underlying tumor development and progression, and the identification of potential therapeutic targets for the treatment of breast cancer. KPL4 cells have several properties that make them useful for research. Firstly, they are relatively easy to culture in the laboratory and can be grown in large quantities. Secondly, they express markers of breast cancer, such as estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2), and exhibit several features characteristic of breast cancer, including uncontrolled proliferation, invasion, and metastasis. Thirdly, they respond to various stimuli, such as hormones and growth factors, making them a useful model system for studying the effects of these factors on breast cancer cell growth and survival.
Breast cancer represents an increasing public health problem as this is the most common cancer in females worldwide, with nearly 1.7 million of new cases detected annually. The KPL-4 cell line was established using the malignant pleural effusion of a breast cancer patient with skin metastasis and has been extensively employed in breast cancer research. According to a 1999 study, published in British Journal of Cancer, the KPL-4 cell line is tumorigenic and readily metastasizes into the lymph nodes and lungs. KPL-4 cells express Erb-B family receptors and have proven to be invaluable models for studying the cell biology of aggressive breast cancers. The KPL-4 model of breast cancer has been used in many studies including one by Suzuki et al. (2009) where the group investigated novel therapies to overcome mutation-mediated resistance to inhibitors of epidermal growth factor receptor (EGFR) kinase, which are a common clinical therapy regimen. Results showed potential for the dual kinase inhibitor MP-412 (aka AV-412), which targets EGFR and ErbB2, and found that it inhibited tumor growth in vivo in otherwise resistant solid tumor models. A 2009 study by Sheuer et al. used KPL-4 to examine the combination treatment of trastuzumab and pertuzumab against human epidermal growth factor receptor 2 (HER2) positive metastatic breast cancers; their results suggested that this combination was a success in inhibiting tumor growth and inducing tumor regression in vivo because of the differing mechanisms of action between the compounds of inhibition of HER2 dimerization (trastuzumab) and prevention of p95HER2 formation (pertuzumab). Finally, Pastuskovas et al. released a study in 2012 investigating the pharmacokinetics of combination treatment of trastuzumab (HER2 inhibitor) and bevacizumab (VEGF inhibitor); they looked into autoradiography, biodistribution and SPECT-CT imaging in a KPL-4 xenograft tumor model and showed that bevacizumab prevented uptake of trastuzumab into tumors which would prevent a favorable clinical outcome, serving as a caution when designing combination therapies and dosing strategies. The KPL4 cell line is used to create the xenograft mouse model. Examination of antitumor activity of monotherapies or combination studies in the KPL4 xenograft model include erlotinib, capecitabine or anti-ErbB2 agents.
KPL4 Orthotopic and Subcutaneous Xenograft Model: Download ![]()
Download Altogen Labs KPL-4 Xenograft Model PowerPoint Presentation: ![]()
Studying Tumor Progression with the Orthotopic KPL-4 Model
The orthotopic KPL-4 model is valuable in cancer research, specifically for studying HER2-positive breast cancer in a more biologically relevant environment. In this model, KPL-4 cells, derived from human breast carcinoma, are implanted directly into the mammary fat pad of immunocompromised mice. This setup allows tumors to develop in their natural location, which mimics the growth patterns and metastatic behavior seen in human breast cancer. The orthotopic KPL-4 model is particularly useful for evaluating the effectiveness of HER2-targeted therapies, as well as investigating tumor progression, metastasis, and the microenvironment’s role in cancer growth. Researchers can use this model to assess drug efficacy, resistance mechanisms, and the interactions between tumor cells and surrounding tissues. It provides a more accurate representation of the complexities of human breast cancer and is instrumental in advancing personalized therapeutic strategies.
Basic study design
- Exponential growth of cells are under aseptic conditions prior to collection.
- Cells are collected for inoculation and viability determined. Trypan blue exclusion is utilized with a 99% minimum cell viability required.
- Cell suspensions are adjusted such that 100 µL of the Matrigel+KPL4 cell suspension contains 1 – 3 million cells. Cells are inoculated s.c. into the flank of a hind leg per mouse. The mice are NOD/SCID or NSG and are 10 to 12 weeks old.
- Calipers are used for tumor monitoring, with expectation of 125-175 mm3 tumors needed to start the study. Animals are sorted into cohorts and compounds are administered according to treatment schedule.
- Daily tumor measurements mouse weights are recorded until the tumor size limit is reached. Tumor dissection performed to remove tumors, record their weight and document digitally.
- Tissues are submersed in RNA-later, snap frozen, nucleic acids isolated or tissues prepared for histological analysis.
Get Instant Quote for
KPL-4 Xenograft Model
KPL-4 and HER2 Signaling: A Model for Studying Tumor Progression and Therapy Resistance
KPL-4 is a human breast cancer cell line that exhibits strong oncogenic characteristics due to its overexpression and amplification of ErbB family receptors, particularly ErbB-2 (HER2). Flow cytometric and dot blot hybridization analyses indicate a 15-fold amplification of the erbB-2 gene, contributing to aggressive tumor growth and progression. The cell line also expresses ErbB-1 (EGFR) and ErbB-3, enabling complex receptor interactions that drive proliferation and survival signaling. Under serum-supplemented conditions, all ErbB receptors undergo autophosphorylation, reinforcing the hyperactive oncogenic signaling in KPL-4 cells. KPL-4 cells produce high levels of interleukin-6 (IL-6), which is implicated in promoting tumor-induced cachexia and immune evasion. Elevated IL-6 levels in culture medium and mouse serum correlate with tumor weight, suggesting a role in systemic disease progression. Additionally, KPL-4-derived tumors in athymic nude mice exhibit rapid growth and occasional lymph node and lung metastases, making them a robust model for studying HER2-driven breast cancer. The sensitivity of KPL-4 to anti-HER2 therapy, such as the monoclonal antibody rhuMAbHER2 (trastuzumab), has been demonstrated, though the response is modest in vivo. This highlights the need for combination strategies to effectively target HER2-overexpressing and IL-6-secreting tumors.
The Role of KPL-4 in HER2-Driven Cancer Studies
KPL-4 is a human breast cancer cell line widely used in research due to its overexpression of HER2 (ErbB-2), a key oncogenic driver. It also expresses EGFR (ErbB-1) and ErbB-3, forming a signaling network that promotes aggressive tumor growth through sustained autophosphorylation and activation of downstream pathways. Notably, KPL-4 produces high levels of pro-inflammatory cytokines and angiogenic factors, contributing to tumor progression and metastasis. This cell line has been instrumental in evaluating HER2-targeting antibody-drug conjugates (ADCs), particularly in assessing drug efficacy and bystander effects in heterogeneous tumor populations. Studies using KPL-4 have provided critical insights into how treatments like DS-8201a can impact both HER2-positive and HER2-negative tumor cells. Given its robust tumorigenic properties and responsiveness to targeted therapies, KPL-4 remains essential in advancing HER2-directed cancer treatments.
KPL-4 Cell Line Validates Novel DVD-ADC Efficacy
In a study conducted by Nanna AR, et al., published by Nature Communications, researchers explored the efficacy of an anti-HER2 dual-variable-domain antibody-drug conjugate (DVD-ADC) in treating HER2-positive breast cancer using the KPL-4 cell line as a model. KPL-4, a human breast cancer cell line expressing HER2, was utilized in both in vitro and in vivo experiments to evaluate the cytotoxicity and therapeutic potential of the novel DVD-ADC. In vitro assays showed that KPL-4 cells were highly sensitive to the ADC, demonstrating a low IC50, indicating potent anti-cancer activity. In vivo, KPL-4 cells were xenografted into NSG mice, and tumor growth inhibition was assessed following ADC administration. Mice receiving the DVD-ADC exhibited significant tumor regression compared to controls and unconjugated antibody groups, with a dose-dependent effect observed at both 5 mg/kg and 10 mg/kg. Notably, survival rates in the 10 mg/kg group were superior to those treated with the benchmark T-DM1 ADC, with some mice achieving complete tumor remission. Further analysis confirmed that the ADC retained its cytotoxic potential after prolonged plasma incubation, highlighting its stability. KPL-4 was instrumental in demonstrating the high specificity and potency of this HER2-targeting ADC, reinforcing its potential as a therapeutic agent for HER2-positive breast cancers. These findings suggest that DVD-ADCs could be a promising alternative to existing HER2-targeted therapies, particularly for cases resistant to T-DM1.
Another study by Liapis E, et al., published by Theranostics journal used eigenspectra multispectral optoacoustic tomography (eMSOT) to investigate the effects of Docetaxel (docetaxel) on breast tumor oxygenation and vascular function, using KPL-4, a HER2-positive breast cancer cell line, as a model. The researchers performed longitudinal imaging over 41 days to assess the impact of Docetaxel on tumor hemodynamics. KPL-4 tumors treated with Docetaxel exhibited a significant reduction in whole tumor oxygenation, as detected by eMSOT imaging. Dynamic oxygen-enhanced eMSOT (OE-eMSOT) revealed a diminished vascular response in Docetaxel-treated KPL-4 tumors, indicating compromised blood flow and increased vascular permeability. Histological validation confirmed that Docetaxel-treated tumors had reduced microvessel density and higher hypoxic fractions compared to controls. Additionally, some KPL-4 tumors developed hemorrhagic blood pools (vascular lakes), which were filled with deoxygenated blood and showed minimal response to oxygen challenge. These findings highlight the anti-angiogenic and vascular-disruptive effects of Docetaxel in KPL-4 tumors, which contribute to tumor hypoxia and potentially influence treatment resistance. The study suggests that eMSOT could serve as a valuable non-invasive method for monitoring tumor microenvironmental changes in response to chemotherapy. The results also have translational implications for optimizing chemotherapy schedules and combining Docetaxel with oxygen-enhancing or hypoxia-targeting therapies.
Following options are available for the KPL-4 xenograft model:
- KPL-4 Tumor Growth Delay (TGD; latency)
- KPL-4 Tumor Growth Inhibition (TGI)
- Dosing frequency and duration of dose administration
- Dosing route (intravenous, intratracheal, continuous infusion, intraperitoneal, intratumoral, oral gavage, topical, intramuscular, subcutaneous, intranasal, using cutting-edge micro-injection techniques and pump-controlled IV injection)
- KPL-4 tumor immunohistochemistry
- Alternative cell engraftment sites (orthotopic transplantation, tail vein injection and left ventricular injection for metastasis studies, injection into the mammary fat pad, intraperitoneal injection)
- Blood chemistry analysis
- Toxicity and survival (optional: performing a broad health observation program)
- Gross necropsies and histopathology
- Positive control group employing cyclophosphamide, at a dosage of 50 mg/kg administered by intramuscular injection to the control group daily for the study duration
- Lipid distribution and metabolic assays
- Imaging studies: Fluorescence-based whole body imaging
