HS578T cell line (human breast cancer cells) was originally isolated from female patient with metastatic breast cancer. These cells are triple-negative – they lack the expression of the estrogen receptor, progesterone receptor, and HER2 protein. HS578T cells are commonly used in biomedical research to study breast cancer biology, metastasis, drug resistance, and the development of new therapies. HS-578 cells are known to be highly invasive and metastatic, making them a valuable tool for studying the mechanisms of breast cancer progression and identifying potential targets for therapeutic intervention. Breast cancer remains the second primary cause of cancer deaths in females as well as the most commonly diagnosed malignancy in women. Well-characterized breast cancer cell lines have proven to be essential tools for investigating the biological characteristics of carcinomas. Preclinical studies of the Hs578T mouse xenograft model are invaluable in examining cellular response to therapeutic drugs and vital for patients with advanced stage disease.
HS578T Breast Cancer Xenograft Model: Download ![]()
Download Altogen Labs Hs578 Xenograft Model PowerPoint Presentation: ![]()
Orthotopic HS578T Xenograft Model
The orthotopic HS578T model involves implanting HS578T breast cancer cells directly into the mammary fat pad of immunocompromised mice, closely mimicking the natural tumor microenvironment and allowing for the study of tumor growth, metastasis, and therapeutic responses. This model is particularly valuable for investigating the progression of triple-negative breast cancer, as HS578T cells are known for their aggressive and metastatic properties. By growing and spreading in ways that resemble human breast cancer, the orthotopic model is ideal for evaluating the efficacy of novel therapies, including chemotherapies and targeted treatments. It also offers insights into drug resistance and immune response dynamics within the tumor microenvironment, making it essential for studying the interaction between tumor and immune cells. Furthermore, the orthotopic HS578T model allows for the exploration of metastatic behavior, providing critical information on the molecular mechanisms behind tumor spread to distant organs. Despite its complexity, this model remains a crucial platform in preclinical research, helping to guide the development of more effective breast cancer treatments.
Get Instant Quote for
HS578T Xenograft Model
TNBC and HS578T Xenograft Model
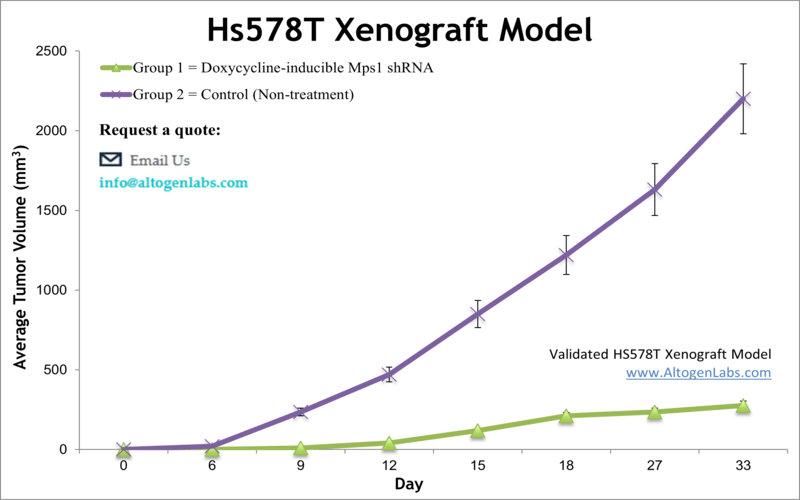
TNBC comprises nearly 10–15 percent of all breast malignancies and has a poor outcome compared to the other types of breast cancer, as per a 2010 article published in Breast Disease. Studies that use the Hs578T xenograft model include the 2016 Nature study by Kim et al. which identified an oncogenic signaling axis involving TrkC, which through downstream regulators promotes primary tumor growth, tumor self-renewal, metastasis, epithelial to mesenchymal transition (EMT) and autocrine-mediated maintenance of the mesenchymal state. Zhang et al. (2014) used the Hs578T mouse xenograft as a TNBC model to examine efficacy and resistance for inhibition of mTOR, which is commonly activated in TNBC patients. They tested two mTOR inhibitors, sirolimus (rapamycin) and temsirolimus (CCI-779), and concluded the need for combination therapy as the inhibitors were successful in limiting growth but not eradicating tumors. Finally, Chen et al. (2018) used the Hs548T xenograft model to verify the role of Contactin 1 (CNTN1) in breast cancer, as it had been implicated in invasion and metastasis in other cancer types. They concluded that CNTN1 promotes proliferation, cell cycle progression, colony formation, migration and invasion in vitro and enhances tumor growth in vivo, making CNTN1 a target for further mechanistic and therapeutic studies. The Hs578T xenograft model enables studies that focus on therapeutics targeting expression mechanisms that control aneuploidy (reduction of Mps1 gene) or degradation of the basement membrane and extracellular matrix that allow higher invasion (MMP-1 and MMP-3 genes).
HS578T Driving Metastasis and EMT in Breast Cancer
In a study conducted by Kim, M., et al., published by Scientific Reports journal, researchers investigated the role of TrkC in metastatic breast cancer, and its ability to promote tumor progression and epithelial-mesenchymal transition (EMT). HS578T, a basal-like breast cancer cell line, was found to express high levels of TrkC, linking it to increased metastatic potential and tumor aggressiveness. The study revealed that TrkC stabilizes JAK2 by inhibiting SOCS3-mediated degradation, leading to the sustained activation of the JAK2/STAT3/Twist-1 axis. This pathway enhances mesenchymal characteristics in HS578T cells, reinforcing their invasive and metastatic behavior. Additionally, TrkC increases IL-6 secretion, forming an autocrine loop that maintains tumorigenicity and resistance to apoptosis. Knockdown of TrkC in HS578T cells significantly reduced their migratory and invasive capabilities, as well as tumor formation in vivo. These findings suggest that TrkC plays a crucial role in driving metastasis and may serve as a potential therapeutic target in aggressive breast cancers.
CNTN1 and Its Role in HS578T Breast Cancer Cell Growth and Metastasis
HS578T is a human triple-negative breast cancer (TNBC) cell line known for its aggressive phenotype, characterized by high proliferative capacity, invasion, and migration potential. Research has identified Contactin-1 (CNTN1) as a key regulator in these processes, with overexpression leading to enhanced tumor growth and metastatic capabilities. CNTN1 promotes cell cycle progression, increasing proliferation and colony formation in vitro while also driving tumor expansion in vivo. Additionally, its upregulation facilitates cell migration and invasion, suggesting a role in breast cancer metastasis. Targeting CNTN1 may offer a promising therapeutic approach for aggressive TNBC, as its inhibition has been linked to reduced tumor progression.
HS578T and PHGDH: Exploring Metabolic Vulnerabilities in Cancer
The HS578T human breast cancer cell line commonly used in oncology research, as they exhibit aggressive growth characteristics and lack estrogen receptor (ER), progesterone receptor (PR), and HER2 expression, making them a valuable model for drug development targeting TNBC. HS578T cells have been shown to rely heavily on the serine synthesis pathway, with high expression of phosphoglycerate dehydrogenase (PHGDH), a key enzyme in this metabolic process. The regulation of PHGDH in HS578T cells is influenced by Parkin, an E3 ubiquitin ligase, which suppresses PHGDH activity through ubiquitination, thereby inhibiting serine synthesis and tumor proliferation. Studies indicate that PHGDH inhibition can significantly reduce the growth of HS578T-derived tumors, making it a potential therapeutic target. Furthermore, research suggests that the loss of Parkin in these cells contributes to metabolic vulnerabilities, highlighting the importance of targeting metabolic pathways in cancer therapy.
Basic study design
1. Prior to collection, all cells are maintained at exponential growth levels.
2. The cells are collected by trypsinizing in the flasks. Cell count is then determined and viability established by trypan blue exclusion (99% min viability).
3. One million cells (Matrigel + Hs578T suspension), in a volume of 150 µL is injected s.c. into rear flank of each mouse (NOD/SCID or athymic BALB/C, 10 to 12 weeks).
4. Injection sites observed until tumors are established.
5. Daily tumor measurements are logged and mouse weights documented (up to 3 times a week).
6. End of study necropsies are performed. Tumors are removed from the study animals and weights recorded.
7. A list of predetermined tissues are collected by standard gross necropsy and either 1) snap frozen, 2) submersed in RNA-later reagent, 3) nucleic acids isolated, or 4) prepared for histological analysis.
Animal handling and maintenance at the Altogen Labs facility is IACUC-regulated and GLP-compliant. Following acclimatization to the vivarium environment, mice are sorted according to body mass. The animals are examined daily for tumor appearance and clinical signs. We provide detailed experimental procedures, health reports and data (all-inclusive report is provided to the client that includes methods, results, discussion and raw data along with statistical analysis). Additional services available include collection of tissue, histology, isolation of total protein or RNA and analysis of gene expression. Our animal facilities have the flexibility to use specialized food or water systems for inducible gene expression systems.
Following options are available for the Hs578T xenograft model:
- Hs578T Tumor Growth Delay (TGD; latency)
- Hs578T Tumor Growth Inhibition (TGI)
- Dosing frequency and duration of dose administration
- Hs578T tumor immunohistochemistry
- Alternative cell engraftment sites (orthotopic transplantation, injection into the mammary fat pad)
- Blood chemistry analysis
- Toxicity and survival
- Gross necropsies and histopathology
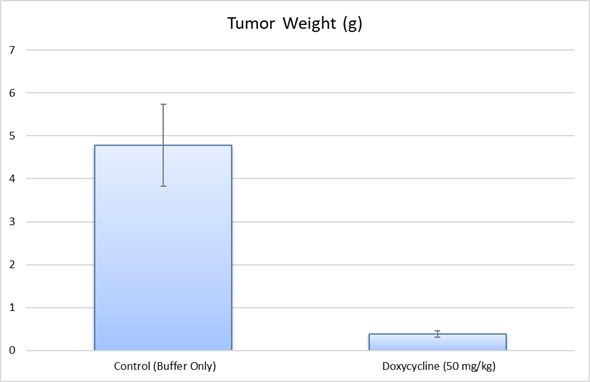
- Positive control group employing dox or cyclophosphamide (20-50 mg/kg)
- Lipid distribution and metabolic assays
