4T1 syngeneic murine model (subcutaneous, orthotopic and metastatic)
Breast cancer remains the second leading cause of cancer deaths in females worldwide. Although necessary improvements in breast cancer diagnosis and management have been made, early detection and anti-metastatic treatment of breast cancer remain a crucial challenge. Preclinical studies of the 4T1 metastatic mouse syngeneic model can help overcome these limitations, which is critical for patients with advanced stage disease. The 4T1 tumorigenic epithelial cell line is derived from the mammary gland tumor of a mouse and is resistant to 6-thioguanine without mutagen treatment. According to a 1992 article in Cancer Research, when injected into BALB/c mice, the 4T1 cell line can spontaneously metastasize to both the lung and liver and form visible nodules in these organs. The 4T1 cell line is particularly useful for stage IV human breast cancer research since tumor from this cell line mimics human breast cancer and readily metastasizes through mice, making a suitable breast cancer model. Liu et al. (2014) used an orthotopic 4TI syngeneic murine model to demonstrate inhibition of the Fas signaling cascade suppresses tumor metastasis and growth and reduces Fas-initiated inflammation in breast cancer, thereby establishing Fas signaling as a potential therapeutic target. A Cell study (Aceto et al., 2014) used a 4T1 syngeneic murine allograft model to define that circulating tumor cell clusters (CTC clusters) present in cancer patients’ blood originate from primary tumors and greatly contribute to metastatic spread of the cancer. Gao et al. (2011) used a murine 4T1 model to test breast cancer formation and metastasis after treatment with doxorubicin (DOX) delivered via pH-sensitive micelles. They found that a polymeric micelle formed with both poly(L-lactide) (PLLA) (Mn 3000)-b-poly(ethylene glycol) (PEG) (Mn 2000)-folate and poly(L-histidine) (PHis) (Mn 4700)-b-PEG (Mn 2000) loaded with DOX prevented metastasis and prevented tumor growth, thereby reporting a novel method for the safer delivery of a known chemotherapy agent. Upon implantation, the production of colony stimulation factors, cytokines, chemokines and angiogenesis factors allows the 4T1 tumor cells to metastasize efficiently. The 4T1 metastatic model enables the study of late-stage breast cancer therapeutic agents.
4T1 Allograft Tumor Model: Download ![]()
Download Altogen Labs 4T1 Syngeneic Murine Model PowerPoint Presentation: ![]()
Oncogenes in 4T1 Tumors
The 4T1 murine breast cancer model harbors a distinct oncogenic landscape that mirrors human triple-negative breast cancer (TNBC). Key oncogenic mutations in Trp53 and Pik3g drive tumor growth and survival, while the absence of mutations in Brca1 and Brca2 differentiates it from hereditary breast cancer models. Highly expressed markers such as Top2a, Birc5, and Mki67 indicate robust proliferative potential, whereas elevated levels of Msln, Ect2, and Plk1 highlight its aggressive metastatic behavior. The integration of the murine mammary tumor virus (MMTV) further influences oncogene expression, particularly through Fgfr2, which is implicated in cancer cell survival and progression. Additionally, 4T1 cells exhibit mutations in Nav3, Cenpf, Muc5Ac, and Gas1, genes linked to tumorigenesis and immune evasion. With a high expression of Gpa33 and Epcam, traditionally associated with gastrointestinal malignancies, 4T1 tumors demonstrate a unique antigenic profile. This comprehensive oncogenic landscape makes 4T1 a powerful model for studying metastatic breast cancer and testing novel therapeutic interventions.
Get Instant Quote for
4T1 Allograft Model
Chemotherapy: Harnessing 4T1 Tumors to Evaluate Immune Checkpoint Therapy
The 4T1 murine breast cancer model plays a pivotal role in evaluating a novel hydrogel-based immunotherapy, PEIGel, designed for intratumoral drug delivery. This hydrogel integrates polyethylenimine (PEI), a polymer with inherent immune-stimulating properties, and magnesium ions to enhance immune response modulation. Research has shown that in 4T1 tumors, PEIGel demonstrated the ability to convert an immunosuppressive tumor microenvironment into an immune-responsive state by increasing PD-L1 expression and promoting M1 macrophage polarization. Encapsulation of anti-PD-L1 antibodies within PEIGel enabled prolonged release, significantly enhancing immune checkpoint blockade (ICB) therapy against 4T1 tumors. Notably, in vivo studies showed that PEIGel administration reduced both primary tumor growth and distant metastases while also preventing tumor relapse post-surgery. The study suggests that PEIGel not only acts as a drug carrier but also actively modulates tumor metabolism and immune response, presenting a promising strategy for localized cancer immunotherapy.
The Impact of Obesity on 4T1 Oncogenic Pathways and Immune Evasion
The 4T1 murine breast cancer model demonstrates a strong oncogenic profile that is significantly influenced by obesity, leading to enhanced tumor growth and metastasis. Research has found that high-fat diet-induced obesity promotes metabolic dysfunction, increasing levels of leptin and inflammatory cytokines that fuel tumor proliferation. 4T1 oncogenes drive aggressive behavior, with increased expression of CXCR4 and CCR9 enhancing tumor cell migration through chemokine gradients. In obese mice, 4T1 cells show a higher capacity for colonizing metastatic niches, particularly within lymph nodes and bone marrow, where immune cell infiltration is compromised. This environment favors immune evasion, as tumor-draining lymph nodes exhibit reduced CD8+ T cell presence and impaired antigen presentation by dendritic cells. Additionally, obesity disrupts bone marrow-derived cytokine signaling, further impairing hematopoiesis and immune responses against tumors. These findings highlight the interplay between metabolic alterations, immune suppression, and oncogene-driven metastatic potential in 4T1 breast cancer cells, offering insights into obesity’s role in cancer progression and potential therapeutic targets.
Advancements in Preclinical Studies Using the Orthotopic 4T1 Model
The orthotopic 4T1 model involves the injection of 4T1 tumor cells into the mammary fat pad of BALB/c mice, replicating the natural site of tumor development in humans. This model closely mimics the biological characteristics of human breast cancer, including tumor growth, invasiveness, and metastasis. Tumors in this model grow in the same tissue environment as in human patients, providing a more physiologically relevant context for studying tumor biology and therapeutic responses. The 4T1 cells used in the orthotopic model exhibit spontaneous metastasis, particularly to the lungs, liver, and bones, making it an effective model for studying metastatic progression. Researchers can monitor tumor growth and metastasis in real-time using imaging techniques such as bioluminescence or magnetic resonance imaging (MRI). Additionally, this model is widely used to evaluate the efficacy of novel therapies, including chemotherapy, targeted treatments, and immunotherapies, offering insights into how these treatments might perform in clinical settings. The orthotopic 4T1 model provides researchers insight when studying the complex interactions between tumors and their microenvironment, providing a platform for preclinical testing of new breast cancer therapies.
Understanding Metastatic Progression with the 4T1 Model
The metastatic 4T1 model is a highly effective system for studying the progression of breast cancer metastasis, as 4T1 cells are known to spontaneously spread to distant organs such as the lungs, liver, and bones. In this model, 4T1 cells are typically implanted either orthotopically or subcutaneously in BALB/c mice, and the resulting tumors closely mimic the metastatic behavior seen in human breast cancer. The model is valuable for understanding the molecular mechanisms driving metastasis, as it allows researchers to study tumor cell invasion, colonization of distant tissues, and interactions with the host immune system. By monitoring the spread of the tumor cells, either through imaging technologies like bioluminescence or through histological examination, researchers can assess the efficacy of novel anti-metastatic therapies. The metastatic 4T1 model is widely used to evaluate drugs that aim to block metastasis, such as chemotherapeutic agents, targeted therapies, and immunotherapies. It has been employed to test treatments that target both primary tumor growth and secondary tumor spread, offering a comprehensive approach to studying therapeutic interventions. Given its reproducibility and relevance to human disease, the metastatic 4T1 model is crucial in advancing our understanding of metastatic breast cancer and for developing more effective treatments.
Basic study design
- Flasks of 4T1 cells are maintained under conditions of exponential growth prior to injection.
- 4T1 cells are prepared for injection by trypsinization and viable cell counts are determined using trypan blue exclusion (98% cell viability required). Cell suspension adjusted to appropriate density.
- Each mouse (NOD/SCID, 10-12 weeks old) receive a subcutaneous injection of one million cells in the flank of the hind leg in a volume of 100 microliters of the Matrigel 4T1 cell suspension.
- The injection sites are palpated three times weekly until tumors are established. Tumors are then measured using digital calipers until they reach an average size of 50-150 mm3.
- Animals are randomized into treatment cohorts and administration of the compound of interest is performed according to the treatment schedule.
- Tumors are measured daily and mouse weights are recorded 2-3 times weekly.
- Animals are euthanized when tumor size reaches 2,000 mm3 or the predetermined size limit per approved IACUC protocol.
- Necropsy and tissue collection are performed as defined for termination of experiment.
- Tumors are excised, weighed and documented by digital imaging.
- Standard gross necropsies are performed and tissues are collected for downstream analysis.
- Tumors and tissues can be snap frozen in LN2, stabilized in RNA-later reagent or prepared for histology.
Metastatic Models
CDX models are mouse allografts used in pre-clinical therapeutic studies. However, as primary tumors proliferate they invade surrounding tissue, become circulatory, survive in circulation, implant in foreign parenchyma and proliferate in the distant tissue. This result leads to an extremely high percentage of death in cancer patients due to metastasis. Metastatic tumor mouse models are utilized to develop novel therapeutic agents that target metastasis (anti-metastatic therapeutics).
To create a metastatic model, the cell line of interest is transfected with vectors containing green fluorescent protein (GFP) or luciferase. Maintained under antibiotic selection, only cells containing the integrated vector will survive. The new cell line clones are capable of stably expressing the gene of interest and are used in metastatic mouse model studies. Although each new cell line clone may contain its own inherent difficulties, the new cell line contains the ability to track internal tumor progression via bioluminescence (luciferase fluorescence after injecting luciferin) or fluorescence (GFP). Internal orthotopic and metastatic tumor growth (not palpable) can now be measured throughout the study, enabling a researcher to gain more insight and additional data in contrast to relying on end of study tumor weight measurements.
Case Study: U87-luc Xenograft Model
An example of Altogen Labs contract research study using a luciferase expressing U87-luc cell line to monitor in vivo tumor growth. The same concept of tumor observation using in vivo imaging is incorporated in metastatic tumor models.
Luciferase expressing U87-luc cells were implanted and tumors allowed to grow. Tumor growth was monitored in a Night Owl (Berthold Technologies) imaging system 10 minutes after an intraperitoneal (IP) injection of the luciferin substrate. As seen in the example below, luciferase expression (measured as photons emitted) in the U87-luc model grants the researcher a visual image and quantifiable metric for orthotopic or metastatic tumor progression.
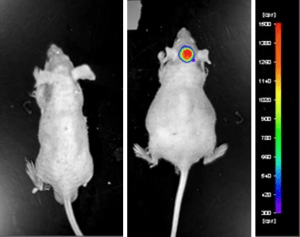
Figure 1. Luciferase expression in U87-luc orthotopic model. Control and implanted glioma mouse model luminescence was analyzed 10 minutes after intraperitoneal luciferin injection.
View full details of the case study by clicking here.
Altogen Labs provides an array of laboratory services using over 100 validated CDX and PDX models. Xenograft and allograft studies can be highly complex, starting with the selection of the appropriate animal model, choice of tumorigenic cell line, administration method, dosing, analysis of tumor growth rates and tumor analysis (histology, mRNA and protein expression levels). Animal handling and maintenance at the Altogen Labs facility is IACUC-regulated and GLP-compliant. Following acclimation to the vivarium environment, mice are sorted according to body mass. The animals are examined daily for tumor appearance and clinical signs. We provide detailed experimental procedures, health reports and data (all-inclusive report is provided to the client that includes methods, results, discussion and raw data along with statistical analysis). Additional services available include collection of tissue, histology, isolation of total protein or RNA and analysis of gene expression. Our animal facilities have the flexibility to use specialized food or water systems for inducible gene expression systems.
Following options are available for 4T1 allograft model:
- 4T1 Tumor Growth Delay (TGD; latency)
- 4T1 Tumor Growth Inhibition (TGI)
- Dosing frequency and duration of dose administration
- Dosing route (intravenous, intratracheal, continuous infusion, intraperitoneal, intratumoral, oral gavage, topical, intramuscular, subcutaneous, intranasal, using cutting-edge micro-injection techniques and pump-controlled IV injection)
- 4T1 tumor immunohistochemistry
- Alternative cell engraftment sites (orthotopic transplantation, tail vein injection and left ventricular injection for metastasis studies, injection into the mammary fat pad, intraperitoneal injection)
- Blood chemistry analysis
- Toxicity and survival (optional: performing a broad health observation program)
- Gross necropsies and histopathology
- Positive control group employing cyclophosphamide, at a dosage of 50 mg/kg administered by intramuscular injection to the control group daily for the study duration
- Lipid distribution and metabolic assays
- Imaging studies: Fluorescence-based whole body imaging
