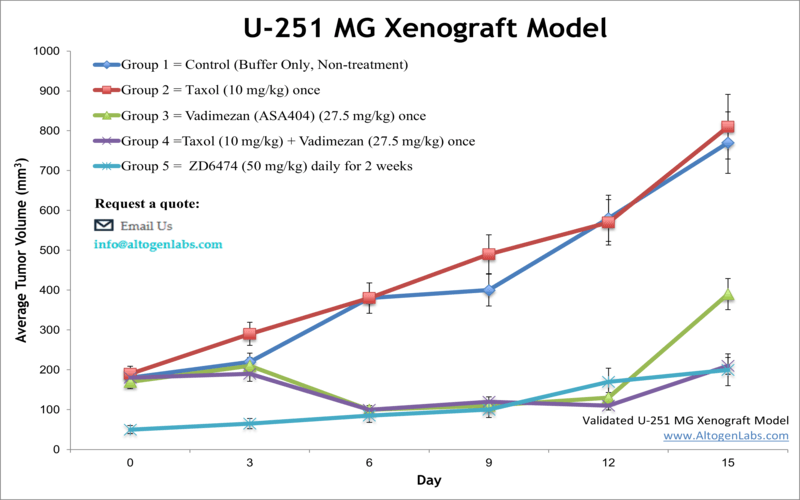
U-251 MG xenograft model
U251MG is a cell line derived from a human glioblastoma multiforme, which is a highly aggressive and malignant brain tumor. An astrocytoma is a form of brain cancer that originates in astrocytes, star-shaped brain cells, and usually remains as a low-grade or benign growth inside the brain and spinal cord not harming other organs. Glioblastoma multiforme (GBM) the most aggressive tumor type, also known as grade IV astrocytoma) and is comprised of several different cell types. Various glioblastoma cancer cell lines have proven to be powerful tools to unravel molecular mechanisms related to brain tumors. The U-251 MG human cell line is derived from a 75-year-old brain cancer patient diagnosed with glioblastoma astrocytoma. Molecular Cancer Therapeutics published a 2017 study (Hilliard et al.) that demonstrated the efficacy of 15α-methoxypuupehenol, an anticancer drug that impacts cell viability of glioblastoma (using the U251 MG line) and breast cancer cells more than normal cells. Hashizume et al. (2010) demonstrated the benefits of using bioluminescence imaging for pre-clinical monitoring and testing of therapeutic regimens for patients with brainstem tumors; one of the xenograft brain tumor models used were U251 MG treated with temozolomide. In 2004, Ozawa et al. published results using U251 MG xenografts to demonstrate the efficacy of GRN163, a telomerase inhibitor, against growth and proliferation of malignant gliomas. It is important to note that the original U-251 cells were established in the 1960s and the modern subclone line (denoted as U-251 MG) have variations in genotype, phenotype and growth characteristics (Torsvik et al., 2014). The U-251 MG cell line is used to create the CDX (Cell Line Derived Xenograft) U-251 MG xenograft mouse model. The U-251 MG xenograft model is a highly invasive pre-clinical model that contains mu-p53 and mu-PTEN. The model yields itself to therapies targeting angiogenesis (e.g. anti-VEGF, vadimezan) and telomerase inhibitors (e.g. GRN163).
U251MG Brain Cancer Xenograft Model: Download ![]()
Download Altogen Labs U251 Xenograft Model PowerPoint Presentation: ![]()
U-251 MG Cell Line
The U-251 MG cell line is a widely used human glioblastoma multiforme model that exhibits key features of aggressive brain tumors, including a mutated p53 gene, robust proliferative capacity, and activation of pro-survival signaling pathways such as NF-kappaB and PI3K/Akt. Its utility in preclinical research spans studies on tumor biology, therapeutic resistance, and apoptosis. U-251 MG cells have demonstrated moderate sensitivity to temozolomide and other chemotherapeutics, with enhanced responses observed when combined with agents that inhibit survival pathways or epigenetic regulators. Both subcutaneous and orthotopic xenograft models derived from U-251 MG have been used to evaluate in vivo tumor growth and treatment response. However, most existing studies focus on single-agent effects and often overlook the interplay between multiple resistance mechanisms or the role of the tumor microenvironment, highlighting a need for more integrative research approaches that address these limitations.
Subcutaneous U-251 MG Xenografts in Glioblastoma Research
Subcutaneous xenograft transplantation using the U-251 MG glioblastoma cell line is a widely employed preclinical approach for studying glioma biology and evaluating novel therapies. Derived from human glioblastoma multiforme, U-251 MG cells reliably form tumors in immunodeficient mice and display key oncogenic features such as invasiveness and resistance to apoptosis. In this model, cells are suspended in Matrigel and injected into the flank of athymic nude or NOD/SCID mice, with tumor growth monitored by caliper measurements to assess treatment response. Although this ectopic model does not replicate the brain microenvironment, it offers advantages in reproducibility, accessibility, and ease of longitudinal evaluation. U-251 MG xenografts have been instrumental in studies targeting DNA repair, cell cycle regulation, and angiogenesis, showing partial sensitivity to temozolomide and enhanced response when combined with agents such as histone deacetylase or checkpoint kinase inhibitors. Despite limitations including the absence of immune and brain-specific stromal interactions, the model remains a robust and scalable platform for preclinical drug screening and mechanistic studies, contributing valuable insights to glioblastoma research.
Advancing Drug Development with Orthotopic U-251 Glioblastoma Models
Orthotopic xenograft transplantation using the U-251 glioblastoma cell line provides a biologically relevant in vivo model for studying tumor behavior within the native brain microenvironment. Derived from a human astrocytoma and marked by p53 mutation, EGFR amplification, and PTEN loss, U-251 cells are stereotactically implanted into the brains of immunodeficient mice, where they form tumors that exhibit invasive growth, angiogenesis, and hypoxia—features consistent with human glioblastoma pathology. Compared to subcutaneous models, orthotopic U-251 xenografts more accurately replicate the spatial constraints and blood-brain barrier challenges encountered in the clinic. Studies employing luciferase-tagged U-251 variants enable longitudinal imaging of tumor development and therapeutic response, while investigations involving convection-enhanced delivery of radiolabeled nanoparticles and small molecule inhibitors have demonstrated the utility of this model in evaluating drug penetration and efficacy. Although limited by the absence of an intact immune system, the orthotopic U-251 model remains a cornerstone for translational glioblastoma research, facilitating mechanistic studies and the preclinical validation of novel therapeutic strategies.
Sulforaphane Regulates Oncogenes to Inhibit U-251 MG Glioblastoma Progression
Sulforaphane, a naturally occurring isothiocyanate found in cruciferous vegetables, exerts significant anticancer effects on U-251 MG glioblastoma cells by inducing apoptosis and inhibiting invasion through modulation of key oncogenic pathways. Cell viability assays demonstrated a clear dose-dependent decrease in U-251 MG survival after 24 hours of sulforaphane treatment, accompanied by morphological changes such as cell shrinkage, membrane blebbing, and nuclear condensation. Flow cytometry confirmed increased rates of apoptosis at concentrations of 20 and 40 micromolar. Western blot analysis revealed that sulforaphane downregulated Bcl-2 and survivin, both of which are known anti-apoptotic proteins highly expressed in glioblastoma, while upregulating pro-apoptotic proteins Bax, Bad, and cytochrome C. The increased Bax-to-Bcl-2 ratio promotes mitochondrial outer membrane permeabilization, leading to cytochrome C release and activation of caspases. The observed decrease in survivin, a marker of poor prognosis and therapeutic resistance in glioblastoma, further supports sulforaphane’s potential to sensitize tumor cells to apoptosis.
In addition to promoting cell death, sulforaphane significantly impairs the invasive behavior of U-251 MG cells. Transwell invasion assays showed a marked reduction in the number of invading cells following treatment, correlating with altered expression of proteins associated with motility and extracellular matrix degradation. Specifically, sulforaphane increased levels of E-cadherin, a tumor suppressor involved in cell adhesion, and reduced expression of Galectin-3, MMP-2, and MMP-9, which are commonly overexpressed in glioblastoma and contribute to matrix remodeling and cell migration. Gelatin zymography confirmed that the enzymatic activities of MMP-2 and MMP-9 were also suppressed. These molecular shifts suggest that sulforaphane disrupts both the structural and signaling components necessary for invasion. Although these findings are limited to in vitro analysis of a single glioblastoma model, they provide compelling evidence that sulforaphane targets multiple oncogenic mechanisms in U-251 MG cells. Further research should investigate its therapeutic efficacy in vivo and evaluate its synergistic potential when combined with standard-of-care treatments.
Basic study design
- U-251 MG cells for inoculation are continually grown under conditions of exponential growth. All cells are prepared for injection using trypsin-EDTA, with viability and cell concentration determined by flow cytometry Guava-PCA cell viability assay. Total cell concentrations are adjusted to 10,000 cells per µL prior to injection.
- T-cell deficient athymic nude mice strain (Foxn1nu/Foxn1+) about 9-12 week old receives a subcutaneous injection (s.c.) in the hind leg of a total of one million cells (50% Matrigel plus U251 MG cell suspension). Injection sites are palpated (tri-weekly) until tumor establishment is determined (100-150 mm3).
- Animals are randomized into treatment cohorts; administration of compounds of interest are dosed according to the treatment schedule.
- Whole body weights and tumor sizes are documented. End of study is marked by tumors reaching the predetermined size limit.
- Necropsies and tissue collections are performed for termination of the study. Tumors are excised & weighed; optional imaging is available.
Get Instant Quote for
U-251 MG Xenograft Model
We provide an array of laboratory services including 120 validated xenograft models (90 standard cell line derived and over 30 PDX models). U251 MG xenograft models can be used to study the biology of glioblastoma, including the mechanisms of tumor growth and metastasis, as well as the tumor’s response to various treatments such as chemotherapy, radiation therapy, and immunotherapy. These models can also be used to evaluate the efficacy and safety of new brain cancer therapies before testing them in human clinical trials. One of the limitations of U251 MG xenograft models is that they may not fully represent the complexity of human brain tumors, as they lack the full complement of immune cells and cellular interactions present in the human body. Moreover, the method of engraftment itself can introduce artifacts that affect the behavior of the transplanted cells. Despite these limitations, U251 MG xenograft models remain an important tool in glioblastoma research for investigating the biology of brain tumors and developing new brain cancer therapies.
Animal handling and maintenance at the Altogen Labs facilities are IACUC-regulated and GLP-compliant. Following acclimation to the vivarium environment, mice are sorted according to body mass. The animals are examined daily for tumor appearance and clinical signs. We provide detailed experimental procedures, health reports and data (all-inclusive report is provided to the client that includes methods, results, discussion and raw data along with statistical analysis). Additional services available include collection of tissue, histology, isolation of total protein or RNA and analysis of gene expression.
U251MG Brain Cancer Xenograft Model: Download ![]()
Following options are available for the U-251 MG xenograft model:
- U-251 MG Tumor Growth Delay (TGD; latency)
- U-251 MG Tumor Growth Inhibition (TGI)
- Dosing frequency and duration of dose administration
- Dosing route (intravenous, intratracheal, continuous infusion, intraperitoneal, intratumoral, oral gavage, topical, intramuscular, subcutaneous, intranasal, using cutting-edge micro-injection techniques and pump-controlled IV injection)
- U-251 MG tumor immunohistochemistry
- Alternative cell engraftment sites (orthotopic transplantation)
- Toxicity and survival (optional: performing a broad health observation program)
- Gross necropsies and histopathology
