U87-LUC xenograft model (Luciferase expressing U-87 cells; subcutaneous and metastatic)
U87Luc cells are a type of human glioblastoma cell line that has been modified to express a luciferase gene. This gene encodes for an enzyme that produces light when it interacts with a substrate, allowing researchers to track the location and growth of U87-Luc cells in real-time using a technique called bioluminescence imaging. Glioblastoma multiforme (GBM) is extremely aggressive brain malignancy, accounting for roughly 60 percent of all brain cancers, with a median survival after diagnosis from 12 to 15 months. U87-luc is a subline of the U-87 MG cell line, isolated in 1966 in Sweden from a 44-year-old Caucasian female patient with Stage 3 glioblastoma. The U87-luc cell line is luciferase modified U-87 MG that has been useful in a variety of bioluminescent imaging studies. A 2014 study by Marrero et al. published in Neoplasia, investigated the anti-cancer activity of serum albumin-binding doxorubicin (Doxo) and aldoxorubicin (Aldoxo) in vivo, using the U87-Luc xenograft model. These findings indicate that anti-tumor efficacy and low toxicity of Aldoxo demonstrate that it could be a potential innovative treatment for GBM patients. A 2007 study by Dinca et al. in the Journal of Neurosurgery used the U87-Luc cell line for bioluminescence imaging to monitor tumor cell growth and response to temozolomide (TMZ) chemotherapy in a rodent model. They concluded this method showed potential for predicting survival and assessing benefits from TMZ treatment which as a methylating agent is a highly toxic therapeutic regimen. A 2017 study used the U87-Luc xenograft tumors to demonstrate the efficacy of using iron oxide nanoparticles and a magnetic hyperthermia technique for a full disappearance of tumors in the mouse models. The U87-luc cell line (human glioblastoma) is used in the creation of the cell line derived U87-luc xenograft mouse model. The U87-luc xenograft has historical significance in the literature as a proven model for assessing angiogenesis and putative anti-angiogenic therapeutic agents.
Basic study design
- All flasks are maintained under aseptic conditions and at an exponential growth phase. The cells are then trypsinized for viability assessment via a trypan blue exclusion assay.
- One million cells (injection volume = 100 µL) are inoculated into each mouse (athymic BALB/c nude, 10 weeks old). Each injection contains a suspension of U87-luc cells plus Matrigel injected subcutaneously in the flank of a hind leg.
- As tumors become established, tumor size is continuously observed. As the average size of the tumors reach average size of 50-150 mm3, animals are sorted into treatment cohorts. Injections of the compound of interest follow the client supplied dosing schedule.
- Daily, tumors are calipered and body weights of the mice are documented. Animals are euthanized when tumor size approaches the study design tumor size limit.
- Final necropsies are performed as defined in the termination of experiment protocol. Immediately following resection, tumors are weighed and digitally imaged. All samples collected can be frozen, prepared in 10% NBF for histology or stabilized (in RNAlater)
- Animals are housed in an animal facility that is pathogen-free in accordance with an established Guide for Care and Use of Laboratory Animals, and also with the regulations of the Institutional Animal Care and Use Committee (IACUC).
Metastatic Model
CDX models are mouse xenografts used in pre-clinical therapeutic studies. However, as primary tumors proliferate they invade surrounding tissue, become circulatory, survive in circulation, implant in foreign parenchyma and proliferate in the distant tissue. This result leads to an extremely high percentage of death in cancer patients due to metastasis. Metastatic tumor mouse models are utilized to develop novel therapeutic agents that target metastasis (anti-metastatic therapeutics).
To create a metastatic model, the cell line of interest is transfected with vectors containing green fluorescent protein (GFP) or luciferase. Maintained under antibiotic selection, only cells containing the integrated vector will survive. The new cell line clones are capable of stably expressing the gene of interest and are used in metastatic mouse model studies. Although each new cell line clone may contain its own inherent difficulties, the new cell line contains the ability to track internal tumor progression via bioluminescence (luciferase fluorescence after injecting luciferin) or fluorescence (GFP). Internal orthotopic and metastatic tumor growth (not palpable) can now be measured throughout the study, enabling a researcher to gain more insight and additional data in contrast to relying on end of study tumor weight measurements.
Case Study: U87-luc Xenograft Model
An example of Altogen Labs research study utilizing a luciferase expressing cell line to monitor orthotopic tumor growth is exhibited below. The same ideology of tumor observation is incorporated in metastatic tumor models.
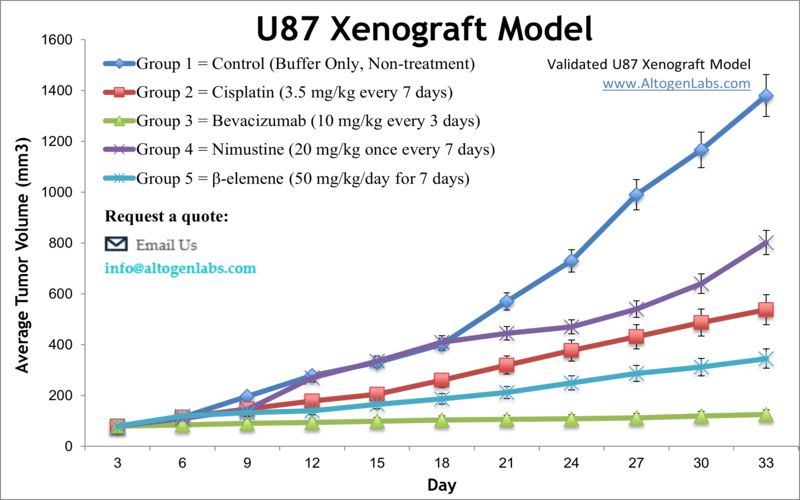
Luciferase expressing U87-luc cells were implanted and tumors allowed to grow. Tumor growth was monitored in a Night Owl (Berthold Technologies) imaging system 10 minutes after an intraperitoneal (IP) injection of the luciferin substrate. As seen in the example below, luciferase expression (measured as photons emitted) in the U87-luc model grants the researcher a visual image and quantifiable metric for orthotopic or metastatic tumor progression.
Figure 1. Luciferase expression in U87-luc orthotopic model. Control and implanted glioma mouse model luminescence was analyzed 10 minutes after intraperitoneal luciferin injection.
Read full text article: [PDF]
Get Instant Quote for
U87-Luciferase Xenograft Model
U87-Luc is a modified version of the U87 human glioblastoma cell line that has been engineered to express luciferase, a bioluminescent protein that emits light in the presence of a specific substrate. This modification allows to monitor the growth and spread of U87-Luc tumors in real-time using bioluminescence imaging. U87-Luc cells have been extensively characterized and are widely used in nonclinical brain cancer research to study the biology of glioblastoma and to test potential cancer therapies. The use of U87-luc cells in brain cancer research has led to numerous advances in understanding of the biology of glioblastoma and the development of new therapies. These studies have contributed to the identification of new therapeutic targets and have provided a foundation for the development of more effective treatments for this devastating disease.
Download Altogen Labs U87 Xenograft Model PowerPoint Presentation: ![]()
Following options are available for the U87-luc xenograft model:
- U87-luc Tumor Growth Delay (TGD; latency)
- U87-luc Tumor Growth Inhibition (TGI)
- Dosing frequency and duration of dose administration
- Dosing route (intravenous, intratracheal, continuous infusion, intraperitoneal, intratumoral, oral gavage, topical, intramuscular, subcutaneous, intranasal, using cutting-edge micro-injection techniques and pump-controlled IV injection)
- U87-luc tumor immunohistochemistry
- Alternative cell engraftment sites (orthotopic transplantation, tail vein injection and left ventricular injection for metastasis studies, injection into the mammary fat pad, intraperitoneal injection)
- Blood chemistry analysis
- Toxicity and survival (optional: performing a broad health observation program)
- Gross necropsies and histopathology
- Positive control group employing cyclophosphamide, at a dosage of 50 mg/kg administered by intramuscular injection to the control group daily for the study duration
- Lipid distribution and metabolic assays
- Imaging studies: Fluorescence-based whole body imaging
