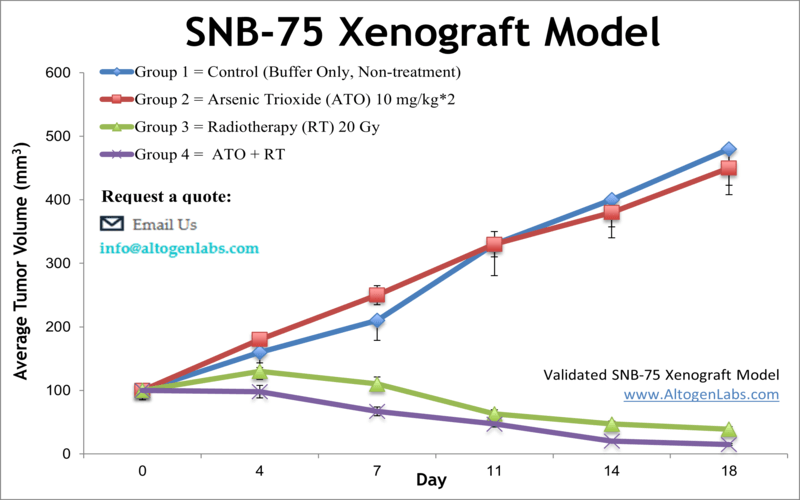
SNB-75 xenograft model
SNB-75 cell line is a human brain tumor cell line. It is also known as SNB-75M. Glioblastoma is the most common type of brain malignancy in adults. It is considered the most aggressive and lethal form of brain tumor, with overall survival of approximately 15 months after diagnosis. Gliomas are the most common type of central nervous system (CNS) malignancy and start in the glial cells (non-neuronal CNS cells) from the brain or spine. Glioma cell lines serve as useful tools for studying the cell biology of brain tumors. The SNB-75 cell line was isolated from a Grade IV glioblastoma obtained in 1980 from a 78-year-old female patient. It is known for being a part of the NCI-60 screening program. In a 1988 study, published in Cancer Research Journal, the SNB-75 cell line was moderately clonogenic in soft agar, showed tumorigenicity in nude mice and secreted plasminogen activator. A 1994 Cancer Research article (Plowman et al.) used SNB-75 cells to study the antitumor activity of temozolomide. The group monitored treatment on SNB-75 xenografted mice and found that there was a synergistic effect between temozolomide and 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU). A 2017 Cancer Research study (Holbeck et al.) used 67 cancer lines, including SNB-75 cells, to evaluate over 5,000 pairings of FDA-approved anticancer drugs. The results were compiled into the NCI-ALMANAC database and was overall useful in identifying clinically relevant anticancer drug combinations. Human tumor xenografting is essential in finding novel treatment therapies for improving the survival rate of brain cancer patients. The SNB-75 cell line is used to create the cell line derived SNB-75 xenograft mouse model. The SNB-75 xenograft model is a functional animal model used to develop novel monotherapies along with combinatorial studies to determine pre-clinical antitumor synergism with temozolomide (e.g. BCNU).
SNB75 Brain Cancer Subcutaneous Xenograft Model: Download ![]()
Download Altogen Labs SNB75 Xenograft Model PowerPoint Presentation: ![]()
Subcutaneous SNB-75 Xenografts in Glioblastoma Research
Subcutaneous xenograft models are a fundamental component of preclinical cancer research, offering a practical and reproducible system for studying tumor growth, drug response, and in vivo tumor biology. Although these models do not replicate the complex brain microenvironment of glioblastoma, they provide a valuable platform for evaluating the tumorigenicity and therapeutic response of glioma cell lines under controlled conditions. The SNB-75 cell line, derived from a human glioblastoma multiforme tumor, has been used effectively in subcutaneous transplantation studies due to its capacity to form consistent tumors when injected into the flank of immunodeficient mice such as athymic BALB/c or NOD/SCID. Injection of one million SNB-75 cells in a Matrigel suspension typically results in tumor formation within 1 to 2 weeks, with volumes reaching 120 to 150 cubic millimeters, at which point treatment can be initiated. These models are often used to evaluate drug efficacy and toxicity, particularly in studies focusing on resistance to alkylating agents like temozolomide. Despite their widespread use, subcutaneous SNB-75 xenografts have inherent limitations. They do not recapitulate key features of glioblastoma progression, such as infiltration, interaction with the neural microenvironment, or the influence of the blood-brain barrier. Nevertheless, these models are indispensable for high-throughput drug screening and initial pharmacokinetic assessments. Their utility can be further enhanced through integration with molecular analyses including RNA sequencing, histopathology, and immunohistochemistry to provide mechanistic insight into treatment response.
SNB-75 as a Model for Fission-Independent Mitochondrial Regulation
Understanding the molecular mechanisms driving glioblastoma progression remains a critical focus in cancer research, with recent studies highlighting mitochondrial dynamics as a potential therapeutic target. In a study published in the International Journal of Molecular Sciences by Kalb RC, et al., the role of Guanylate-Binding Protein-1 (GBP-1) in glioblastoma mitochondrial dynamics was explored, with particular attention to the SNB-75 cell line. The authors demonstrated that GBP-1 localizes to the cytosolic side of the outer mitochondrial membrane in SNB-75 cells but does not induce the characteristic mitochondrial fission seen in other GBM models such as U251. SNB-75 cells maintained elongated, filamentous mitochondria and exhibited low levels of mitochondrial-associated Drp1 despite high GBP-1 expression, indicating a resistance to fission processes typically driven by GBP-1. This contrasted with U251 cells, in which GBP-1 overexpression promoted Drp1 translocation, reduced mitochondrial length, and decreased sensitivity to Drp1 inhibition. These findings reveal a divergence in mitochondrial behavior across GBM cell lines and suggest that GBP-1’s functional effects are context-dependent. The study employed high-resolution confocal microscopy, subcellular fractionation, and isogenic expression models to dissect GBP-1’s influence on mitochondrial structure. While technically rigorous, the absence of in vivo studies using SNB-75-derived xenografts limits broader translational interpretation. Nonetheless, the resistant mitochondrial phenotype observed in SNB-75 cells presents a unique opportunity to study fission-independent mechanisms of glioblastoma cell survival and migration. It remains to be determined whether SNB-75’s resistance arises from altered Drp1 dynamics, cytoskeletal architecture, or mitochondrial membrane properties. These findings encourage future research to investigate regulatory elements that constrain mitochondrial remodeling in certain glioblastoma subtypes, with SNB-75 serving as a valuable model for delineating non-canonical mitochondrial behavior in tumor cells.
CK1 Inhibition Induces Apoptosis in SNB-75 Glioblastoma Cells
A study by Varghese et al., published in Scientific Reports journal, investigates the role of Casein Kinase 1 epsilon (CK1ε) in regulating glioblastoma (GBM) cell survival, with particular emphasis on its activity across multiple GBM cell lines, including SNB-75. The authors report that CK1ε is the most highly expressed isoform among six CK1 family members in GBM, with elevated levels in both tumor tissue and cell lines. Depletion of CK1ε using shRNA resulted in significant viability loss in SNB-75 cells, reducing cell survival to below 10 percent, indicating strong sensitivity. Interestingly, while SNB-75 expresses high levels of CK1ε protein, the study found no correlation between CK1ε abundance and sensitivity to depletion across GBM lines, suggesting that kinase activity, not expression level, is critical. The mechanism of cytotoxicity was traced to CK1ε’s negative regulation of β-catenin; its depletion activated β-catenin signaling, leading to caspase-3-dependent apoptosis in responsive cell lines such as SNB-75. The methodology involved robust in vitro and in vivo assays, including shRNA-mediated knockdowns, caspase activity quantification, luciferase reporter assays, and use of CK1ε inhibitors (IC261 and PF-4800567). The results were consistent across established cell lines, primary GBM lines, and glioblastoma stem cells (GSCs), with IC261 significantly suppressing tumor growth in LN229/GSC xenografts. However, IC261’s low selectivity raises concerns about off-target effects, limiting its translational potential. The use of SNB-75, which showed one of the strongest apoptotic responses upon CK1ε inhibition, underscores its value as a model for dissecting β-catenin-mediated survival pathways. The study positions CK1ε as a non-canonical regulator of β-catenin in GBM, challenging conventional views of β-catenin as solely oncogenic and suggesting it may have context-dependent pro-apoptotic roles. Future research should focus on developing CK1ε-selective inhibitors capable of activating β-catenin-mediated apoptosis, with SNB-75 serving as a critical platform for preclinical validation.
Compound 6h Demonstrates Promising Cytotoxicity Against Glioblastoma
A series of 4-chloro-2-((5-aryl-1,3,4-oxadiazol-2-yl)amino)phenol analogues has demonstrated notable antiproliferative activity across multiple cancer types, with compound 6h emerging as a particularly effective agent. When evaluated against a panel of human tumor cell lines, 6h achieved a percent growth inhibition (PGI) of 54.68 in SNB-75 glioblastoma cells, 65.12 in SNB-19, and 55.61 in the non-small cell lung carcinoma line NCI-H460. These results underscore its promising cytotoxic profile, particularly against central nervous system (CNS) tumor models. The observed activity is likely attributable to the compound’s structural compatibility with the tubulin binding site, as docking simulations revealed that 6h aligns well within the hydrophobic cavity of the tubulin–combretastatin A4 complex, engaging residues such as Leu252, Ala250, and Ala317 through its trimethoxyphenyl moiety. Although strong polar interactions were not detected, the hydrophobic fit appears sufficient to disrupt tubulin dynamics and inhibit cell proliferation. The consistent cytotoxicity of 6h across CNS and other tumor types suggests a broad mechanism of action, likely rooted in its interaction with the colchicine-binding domain of tubulin. However, its efficacy was measured using a single 10 micromolar dose, which limits the capacity to evaluate concentration-dependent effects or calculate IC50 values. This restricts the depth of pharmacological insight and hinders comparisons with established chemotherapeutics. In the case of SNB-75, the moderate PGI suggests meaningful but incomplete growth suppression, highlighting the need for further optimization or combination approaches. Additionally, in vivo validation, particularly in subcutaneous or orthotopic SNB-75 xenograft models, would be critical to assess therapeutic potential under physiologically relevant conditions. Advancing this research will require dose-response profiling, mechanistic assays to assess microtubule integrity, and translational studies that establish efficacy in preclinical glioblastoma models.
SNB-75 Highlights Cell-Specific EMT Responses to Tumor Hypoxia
Hypoxia plays a pivotal role in shaping glioblastoma cell behavior, particularly through its influence on mesenchymal transdifferentiation and invasive capacity. In controlled experiments, SNB-75 cells exposed to hypoxic conditions (1 percent O₂) for 72 hours underwent marked morphological changes, developing an elongated phenotype indicative of mesenchymal transition. Under these same conditions, SNB-75 cells demonstrated upregulated expression of mesenchymal markers such as fibronectin and COL5A1, proteins typically absent in their normoxic state. This phenotypic shift was accompanied by enhanced invasive potential, confirming a functional transition consistent with epithelial-to-mesenchymal transition (EMT)-like behavior. Importantly, this response was not uniform across cell lines; while U87 cells mirrored the behavior of SNB-75, U251 cells did not exhibit comparable changes, emphasizing cell line–specific responses to hypoxia. Mechanistically, this mesenchymal shift was mediated by the stabilization and nuclear translocation of HIF1α and the downstream EMT transcription factor ZEB1. Silencing HIF1α with shRNA inhibited both ZEB1 induction and fibronectin expression, preventing morphological transition and suppressing invasion in SNB-75 analogs. In contrast, knockdown of HIF2α did not produce similar effects, underscoring the specificity of the HIF1α–ZEB1 axis. Additional inhibition using digoxin, a cardiac glycoside known to suppress HIF1α translation, corroborated this finding, as treated cells failed to undergo hypoxia-induced transition. ZEB1 was further confirmed as a necessary downstream effector, as its silencing alone was sufficient to block mesenchymal marker expression and reduce invasion. These observations were validated in patient-derived GBM tissue, where hypoxic regions showed colocalized expression of GLUT1, ZEB1, and YKL40. Collectively, the data illustrate that SNB-75 is a responsive model for dissecting hypoxia-driven invasiveness in glioblastoma and supports therapeutic strategies that target the HIF1α–ZEB1 signaling axis to hinder tumor progression.
Oncogenic Pathways and Immune Evasion in SNB-75 Glioblastoma
A comprehensive multi-pathway analysis of glioblastoma revealed 12 genes, each from one of ten canonical oncogenic signaling cascades, that were significantly associated with patient mortality and disease progression. Using TCGA transcriptomic and survival data, the study found that specific expression levels of these genes correlated with clinical outcomes. Among these, genes such as MDM2, DDB2, PAK1, TNFRSF1A, and MAFF were linked to poor prognosis when highly expressed, while moderate levels of E2F2 and high expression of CTBP2 were associated with better survival outcomes. Importantly, the expression of several poor-prognosis genes negatively correlated with CD8+ T-cell infiltration in the tumor microenvironment, implicating an immunosuppressive role. In GBM models, including SNB-75, which features relevant genetic backgrounds for such pathways, these genes are particularly important for understanding tumor aggressiveness, resistance mechanisms, and immune evasion.
Among the notable findings, the p53 regulator MDM2 and the Wnt-associated DKK3 were positively correlated with each other and with other markers of poor survival such as DDB2 and MAFF, suggesting an interlinked oncogenic network. Conversely, CTBP2, a transcriptional co-repressor that antagonizes Wnt signaling, was associated with favorable outcomes and increased CD8+ T-cell infiltration. The use of in silico flow cytometry and drug sensitivity databases further supported the therapeutic relevance of these genes. For instance, nutlin-3a was shown to inhibit MDM2 and TNFRSF1A expression across multiple GBM cell lines, including SNB-75. These observations indicate that SNB-75 may serve as a valuable model for preclinical evaluation of multi-targeted therapies that modulate these signaling pathways. Future studies should focus on mechanistic validation in xenograft systems and assess the immunological consequences of targeting these oncogenes in immune-competent or humanized models
SNB75 Brain Cancer Subcutaneous Xenograft Model: Download ![]()
Basic study design
- 10 to 12 w.o. athymic BALB/C or NOD/SCID receive single injections into the flank of the hind leg. Each injection contains one million cells of the 50% Matrigel plus SNB-75 cell suspension (in a volume of 0.1 ml).
- The injection sites are watched and palpated until tumors are deemed established. Tumors are assessed (digital calipers) until they reach average sizes in the range of 120-150 mm3. Treatment groups are established by randomization. The compound of interest is injected according to customer supplied dosing schedule.
- In-life tumor measurements are taken daily, with whole body weights tracked 3 times weekly.
- Maximum permitted tumor size determines the end of the study, at which time necropsies and tissue collections are performed.
- Tumor samples are excised and weighed. Tumor samples can also be documented by digital imaging. All tissues collected are stored according to customer indication (snap frozen in liquid nitrogen, immersed in 10% NBF formalin solution, etc).
Get Instant Quote for
SNB-75 Xenograft Model
SNB-75 cells have been extensively characterized and are known to harbor several genetic mutations, including mutations in the tumor suppressor gene p53 and the oncogene EGFR. These mutations are commonly found in glioblastoma tumors and are thought to contribute to tumor growth and resistance to therapy. SNB-75 cells are also known to express high levels of the enzyme carbonic anhydrase 12, which has been implicated in the growth and invasiveness of glioblastoma tumors. Xenograft animal models are used to assess the effectiveness of test compounds against specific types of cancer.
Following options are available for the SNB-75 xenograft model:
- SNB-75 Tumor Growth Delay (TGD; latency)
- SNB-75 Tumor Growth Inhibition (TGI)
- Dosing frequency and duration of dose administration
- Dosing route
- SNB-75 tumor immunohistochemistry
- Blood chemistry analysis
- Toxicity and survival (optional: performing a broad health observation program)
- Gross necropsies and tissue pathology analysis
