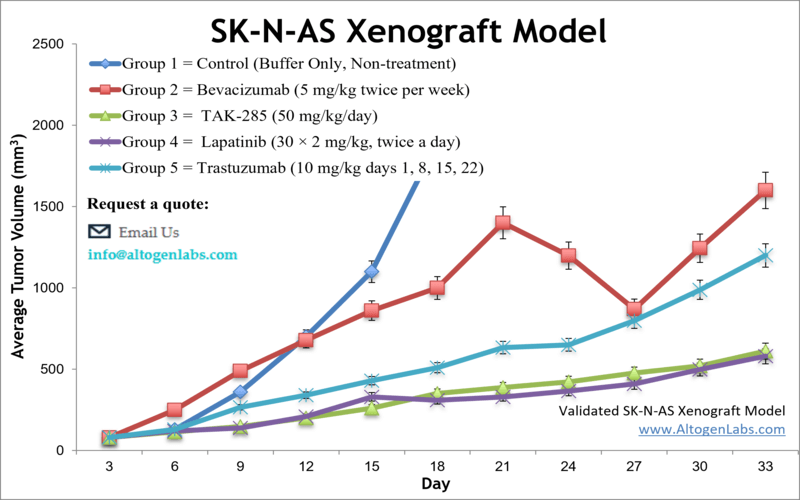
SK-N-AS xenograft model
Neuroblastoma is the most common extracranial solid tumor of childhood, with nearly 50% of patients developing bone metastases that result in significant morbidity, including bone pain and impaired mobility. These metastatic events are strongly associated with poor clinical outcomes, and long-term survival in high-risk neuroblastoma cases remains below 40%. The SK-N-AS cell line is a hyperdiploid neuroblastoma line originally derived from the bone marrow metastasis of a six-year-old female patient. SKNAS cells demonstrate autonomous proliferation driven by autocrine stimulation with insulin-like growth factor 2 (IGF-2). This cell line constitutively produces high levels of IGF-2 and expresses type 1 IGF receptors, contributing to its aggressive phenotype and utility in preclinical cancer research, including studies of brain and bone metastatic tumors. The SK-N-AS xenograft model has been widely used to investigate molecular mechanisms of tumor progression and therapeutic resistance. Polo-like kinase 1 (PLK1), which is overexpressed in a wide range of malignancies including high-risk neuroblastoma, has been implicated in poor patient prognosis. A 2011 study by Ackermann et al. demonstrated significant antitumor efficacy of the PLK1 inhibitor using the SK-N-AS xenograft model. Further supporting its value in metastasis research, a 2014 study published in the Journal of Bone Oncology showed that SK-N-AS cells uniquely induced expression of receptor activator of NF-κB ligand (RANKL), COX-2 mRNA, and secreted elevated levels of prostaglandin E2 (PGE2) – key mediators of osteoclastogenesis and bone metastatic progression. In 2016, a study by Beadry et al. in Pediatric Blood & Cancer used the SK-N-AS xenograft model in conjunction with serum metabolomics to successfully differentiate between high- and low-risk patients, as well as between active disease and partial or complete therapeutic responses.
Altogen Labs offers customizable xenotransplantation services utilizing the SK-N-AS cell line. The in-house validated CDX model created with SK-N-AS cells provides a high-risk neuroblastoma platform with elevated PLK1 expression. SK-N-AS exhibits genetic alterations such as 1p deletion and 11q loss, both of which are linked to poor clinical outcomes. The cell line is p53 wild-type and displays notable resistance to conventional chemotherapies including vincristine and doxorubicin, making it a valuable tool for investigating drug resistance. Prior studies have explored the involvement of anti-apoptotic proteins, hypoxia-inducible factors, and drug efflux transporters in these resistant phenotypes. SK-N-AS has also been used in immune-oncology research, revealing low responsiveness to immune checkpoint blockade and evidence of immune evasion through cytokine secretion and impaired antigen presentation.This model is a powerful research tool for evaluating the efficacy of targeted therapies, including PLK1 inhibitors, as well as commonly used anticancer agents such as lapatinib, trastuzumab, and rosiglitazone.
SKNAS Metastatic And Orthotopic Xenograft Model: Download ![]()
Download Altogen Labs SKNAS Xenograft Model PowerPoint Presentation: ![]()
Subcutaneous SK-N-AS Xenografts in Neuroblastoma Research
Subcutaneous xenograft transplantation using the SK-N-AS neuroblastoma cell line provides a robust and reproducible model for studying high-risk, non-MYCN-amplified neuroblastoma in vivo. SK-N-AS cells, which exhibit chemoresistance, wild-type TP53 expression, and chromosomal alterations including 1p and 11q loss, are injected subcutaneously into immunocompromised mice to generate rapidly growing tumors suitable for pharmacological evaluation. This model has been widely used to investigate drug responses, particularly to Polo-like kinase 1 inhibitors such as BI 2536 and volasertib, which have shown significant anti-tumor activity in preclinical studies. Although the subcutaneous environment does not fully replicate the anatomical context of neuroblastoma, the model remains valuable for its ease of use, high engraftment rates, and compatibility with longitudinal tumor measurements. Our studies have demonstrated that SK-N-AS xenografts display distinct metabolic reprogramming in response to targeted therapies, including alterations in oxidative phosphorylation and glycolysis that may underlie resistance. By integrating histological, transcriptomic, and metabolic analyses, this model serves as an essential platform for preclinical screening, mechanistic discovery, and drug prioritization in early-stage neuroblastoma research.
Modeling Site-Specific Tumor Dynamics with SK-N-AS Orthotopic Xenografts
Orthotopic xenograft transplantation using the SK-N-AS neuroblastoma cell line provides a biologically relevant in vivo model for studying high-risk, non-MYCN-amplified neuroblastoma within its native microenvironment. By injecting SK-N-AS cells into the adrenal gland or paraspinal region of immunodeficient mice, researchers replicate the anatomical context of tumor growth, enabling more accurate evaluation of tumor-stromal interactions, local invasion, angiogenesis, and therapeutic response. Although SK-N-AS has a relatively low metastatic potential compared to MYCN-amplified lines, orthotopic implantation has produced localized tumors and, in some cases, limited metastatic spread. These models have been used to investigate context-sensitive therapies, including anti-angiogenic agents and spatially targeted drug delivery methods, with imaging techniques such as MRI and bioluminescence facilitating non-invasive tumor tracking. While technically demanding, orthotopic SK-N-AS models offer enhanced physiological relevance and have been critical in identifying biomarkers of therapeutic resistance and local progression. When combined with multi-omics analyses, this model significantly contributes to the refinement of neuroblastoma treatment strategies and advances translational cancer research.
SK-N-AS Metastasis Models for Drug Testing
Metastatic xenograft transplantation using the SK-N-AS neuroblastoma cell line provides a vital platform for modeling the systemic progression of high-risk, non-MYCN-amplified neuroblastoma. Although SK-N-AS cells exhibit modest intrinsic metastatic capacity, their injection via intravenous or intracardiac routes into immunodeficient mice has enabled the development of experimental models that recapitulate multi-organ dissemination, including colonization of the liver, lungs, and bone marrow. These models have been instrumental in studying metastatic organotropism, drug distribution, and resistance mechanisms. Transcriptomic analyses of SK-N-AS-derived metastatic lesions have revealed dysregulation of adhesion molecules, chemokine signaling, and epithelial-to-mesenchymal transition pathways associated with invasive behavior. Moreover, they provide a clinically relevant context for evaluating novel therapies targeting residual disease and tissue-specific metastases, particularly in pharmacologically challenging niches such as bone marrow. Our own investigations using metastatic SK-N-AS models have demonstrated site-specific differences in therapeutic response to epigenetic and immunomodulatory agents. While technical variability remains a limitation, these models offer critical insight into the biology of neuroblastoma metastasis and are essential for advancing the preclinical development of anti-metastatic strategies.
COX-2 as a Driver of Neuroblastoma Bone Lesions
The SK-N-AS neuroblastoma cell line serves as a valuable model for investigating bone metastasis in pediatric cancer, offering insight into how tumor cells colonize and degrade bone tissue. When introduced into the circulation of immunocompromised mice, SK-N-AS cells consistently form osteolytic lesions driven by enhanced osteoclast activity, elevated cyclooxygenase-2 (COX-2) expression, and increased secretion of prostaglandin E2 (PGE2). These factors promote both bone resorption and angiogenesis, creating a microenvironment favorable to tumor expansion. Inhibiting COX-2 significantly reduces bone destruction, osteoclast recruitment, and vascular endothelial growth factor (VEGF) expression, highlighting its central role in metastatic progression. Notably, this pathway appears to operate independently of parathyroid hormone-related protein (PTHrP), suggesting mechanistic diversity among neuroblastoma subtypes. While the experimental design offers strong physiological relevance through intracardiac injection and co-culture systems, broader validation across additional cell lines is needed. Overall, SK-N-AS provides a clear framework for understanding tumor-bone interactions and supports further research into COX-2 inhibition as a therapeutic strategy to limit neuroblastoma metastasis and its skeletal complications.
Epigenetic Reprogramming of SK-N-AS Cells
SK-N-AS neuroblastoma cells can be epigenetically reprogrammed to acquire stable stem cell-like properties, offering a valuable model for studying tumor-initiating behavior in non-MYCN-amplified neuroblastoma. Upon transient treatment with DNA methylation inhibitors, SK-N-AS cells upregulate core stemness factors such as SOX2, POU5F1, and KLF4, and express surface markers like CD133 and CXCR4. These induced cells maintain sphere-forming capacity and stem-like characteristics for extended periods in culture without further treatment. In xenograft models, even small numbers of reprogrammed SK-N-AS cells are capable of initiating tumors with high efficiency, producing undifferentiated large-cell neuroblastomas that histologically resemble the most aggressive human cases. This phenotype is marked by large vesicular nuclei, high mitotic index, and uniform cell morphology, features associated with poor clinical prognosis. Several key patterns emerge from this reprogramming model. Increased expression of stemness genes correlates with elevated tumorigenic potential, enhanced MYC protein expression, and aggressive histopathological features. CXCR4, a chemokine receptor often linked to metastatic signaling, is strongly expressed in reprogrammed SK-N-AS tumors but absent in tumors derived from non-reprogrammed cells, suggesting a role in maintaining the tumor-initiating phenotype. Moreover, these reprogrammed cells display dramatically increased sensitivity to heat shock protein 90 (Hsp90) inhibitors, with rapid downregulation of MYC following treatment. This suggests a therapeutic vulnerability that may be exploited in targeting stem-like neuroblastoma populations. While the approach highlights the plasticity of neuroblastoma cells under epigenetic influence, further investigation is needed to clarify the molecular mechanisms that stabilize this phenotype and determine whether similar vulnerabilities exist across diverse genetic backgrounds. This model underscores the importance of epigenetic state in regulating tumor initiation and provides a foundation for developing therapies directed at aggressive, undifferentiated neuroblastoma.
FZD2 Signaling Drives SK-N-AS Tumor Growth
Frizzled2 (FZD2) signaling has emerged as a critical regulator of neuroblastoma cell behavior, particularly in high-risk forms of the disease. In SK-N-AS cells, which lack MYCN amplification, FZD2 influences both β-catenin-dependent and β-catenin-independent Wnt signaling pathways. These dual-signaling axes orchestrate various oncogenic behaviors, including proliferation, migration, and survival. Baseline signaling analysis reveals that SK-N-AS cells exhibit elevated total and active β-catenin, MYC expression, and phosphorylated ERK, in contrast to MYCN-amplified lines which display higher cyclin D1 and LRP6 phosphorylation. Functionally, FZD2 promotes SK-N-AS cell proliferation through canonical Wnt signaling components such as LRP6, β-catenin, and MYC, while also engaging non-canonical effectors like PKC and ERK. siRNA-mediated silencing of FZD2 in SK-N-AS significantly reduces tumor cell proliferation and migration in vitro and suppresses tumor growth in vivo xenografts, accompanied by lower β-catenin activity, decreased Ki67 proliferation indices, and reduced angiogenesis. These findings highlight a complex regulatory network in which FZD2 integrates inputs from multiple Wnt ligands to drive oncogenesis in SK-N-AS cells through distinct molecular cascades. The downregulation of MYC and cyclin D1 upon FZD2 knockdown, along with increased PKC and ERK phosphorylation, suggests a signaling shift that impairs tumor growth. Rac1 activity, a marker of non-canonical pathway engagement, is also attenuated in response to FZD2 blockade, correlating with reduced cell motility. While the study underscores the role of FZD2 as a potential therapeutic target, it also raises questions about pathway compensation and receptor redundancy in the tumor microenvironment. Further research should investigate how targeting FZD2 influences signaling crosstalk, particularly the balance between Wnt-driven proliferation and differentiation programs in neuroblastoma subtypes like SK-N-AS.
Chromosomal Adaptation and Drug Resistance in Neuroblastoma
The SK-N-AS neuroblastoma cell line, which lacks MYCN amplification, provides an instructive model for understanding how drug resistance and oncogene regulation evolve under chemotherapeutic pressure. Upon long-term exposure to cisplatin, SK-N-AS cells (designated SK-N-ASCDDP) exhibit increased MYCN mRNA expression alongside chromosomal alterations, specifically gain of the 2p arm where the MYCN gene resides. This increase in MYCN expression occurs despite the absence of classical gene amplification and appears to result from clonal expansion of subpopulations with extra 2p copies, as visualized by interphase and metaphase FISH. Notably, this gain of 2p was not detected by MLPA, likely due to its averaging of signal across heterogeneous cell populations, underscoring the importance of using multiple methods for genomic analysis. The correlation between MYCN gene copy gain and elevated transcript levels in SK-N-ASCDDP supports the hypothesis that 2p gain confers a selective advantage during cisplatin treatment, potentially facilitating resistance mechanisms.
Patterns in the data further highlight how increased MYCN expression in SK-N-AS cells is not necessarily associated with amplification in the classical sense but may still result in functional consequences for tumor progression and drug resistance. While MYCN amplification is a known marker of poor prognosis, modest increases in copy number such as those seen in 2p gain can still enhance transcriptional activity and influence treatment outcomes. In SK-N-ASCDDP, MYCN overexpression does not appear to result from acute cisplatin exposure but is rather linked to long-term selection pressures, suggesting a cumulative adaptive mechanism. This phenomenon emphasizes the plasticity of neuroblastoma cells in modulating oncogene dosage to survive cytotoxic stress. The study also reinforces that high MYCN expression, even without amplification, can contribute to aggressive tumor behavior when combined with defects in apoptotic regulation. These insights broaden the understanding of MYCN-associated resistance beyond amplification status and underscore the need for therapeutic strategies that target both MYCN transcriptional regulation and genomic stability in neuroblastoma subtypes like SK-N-AS.
Basic study design
- SK-N-AS cells are trypsinized while cell growth is at exponential phase growth. Flow cytometry (Guava PCA) cell viability assay is performed to confirm at least 97-98% cell viability and measure cell concentration. Total suspension concentration is corrected to the cell density needed for injection.
- Athymic nude mice strain (Foxn1nu/Foxn1+) about 10-12 weeks old receive a single 100 µL injection (subcutaneous) in a hind leg containing one million cells (50% Matrigel + SKNAS cell suspension).
- Palpations are performed up to three times a week until tumor establishment. Calipering of tumors are performed to monitor progression of tumor growth. In-life study begins when a size of 50-150 mm3 is reached.
- Animals are grouped into client designed cohorts and the test compounds are administered. Tumors are continuously monitored (daily measurements) along with mouse body weights logged.
- The in-life study is terminated when tumor size reaches the study tumor size limit. As directed by the client, tissues are collected and prepared for histology, frozen or nucleic acids isolated. All resected tumors are digitally imaged and weighed.
Get Instant Quote for
SK-N-AS Xenograft Model
The different types of brain tumor cell lines are astrocytes, neurons, Schwann cell lines, brain endothelial cells, glial, pituitary tumor, retinal, medulloblastoma, and GBM stem cells. Astrocytes are the most abundant cell type in mammalian brains. It shows excellent proliferative ability. It includes glial fibrillary protein. Neuron cells have mature neurons which do not undergo cell division. One way to overcome this is to establish secondary cell lines that are derived from neuronal tumors and have become immortalized. They have the advantage of being grown fairy in cell cultures. Schwann cell lines play an essential role in development, maintenance, function, & regeneration of peripheral nerves. Brain endothelial nerves have the capacity to regulate the passage of the molecules and cells to and from the neural parenchyma and constitute the blood brain barrier. Glial cells, in response to injury, retain the ability to divide. Pituitary tumor cells play a critical role in the development and maintenance of adjacent photoreceptors in the vertebrae retina. Medulloblastoma cells form in the cerebrum, it forms in fetal cells that remain after birth. GBM stem cells differentiate into cells of all three neutral lineages including cells expressing markers of immature neurons. Xenograft animal models are used to assess the effectiveness of experimental test compounds against specific types of cancer. Novel medicines are tested on staged tumor growths that have been engrafted via subcutaneous or orthotopic inoculation in an immunocompromised mouse or rat model (Nude or NOD/SCID). All clinically approved anti-cancer agents have been evaluated with conventional preclinical in vivo models.
SK-N-AS is a human neuroblastoma cell line that is often used in preclinical research as a model for studying the biology of neuroblastoma and testing potential treatments for the disease. SK-N-AS cells were originally derived from a bone marrow metastasis in a patient with neuroblastoma. SK-N-AS xenograft studies performed to study tumor growth and response to treatment in vivo. Xenograft studies can be highly complex, starting with the selection of the appropriate animal model, choice of tumorigenic cell line, administration method, dosing, analysis of tumor growth rates and tumor analysis (histology, mRNA and protein expression levels). Altogen Labs provides an array of laboratory services using over 120 CDX and PDX models. Researchers investigating the role of specific proteins or gene products in regulating tumor growth can benefit from development of protein overexpression (genetically engineered to ectopically express proteins, tumor suppressors, or oncogenes) and RNAi cell lines with long term gene silencing. Animal handling and maintenance at the Altogen Labs facilities are IACUC regulated and GLP-compliant. We provide detailed experimental procedures, health reports and data (all-inclusive report is provided to the client that includes methods, results, discussion and raw data along with statistical analysis).
SK-N-AS Xenograft Model: Download ![]()
Following options are available for the SKNAS xenograft model:
- SK-N-AS Tumor Growth Delay (TGD; latency)
- SK-N-AS Tumor Growth Inhibition (TGI)
- Dosing frequency and duration of dose administration
- Dosing route (intravenous, continuous infusion, intraperitoneal, intratumoral, oral gavage, topical, intramuscular, subcutaneous, intranasal, using cutting-edge micro-injection techniques and pump-controlled IV injection)
- SK-N-AS tumor immunohistochemistry
- Alternative cell engraftment sites (orthotopic transplantation)
- Blood chemistry analysis
- Toxicity and survival (optional: performing a broad health observation program)
- Gross necropsies and histopathology
