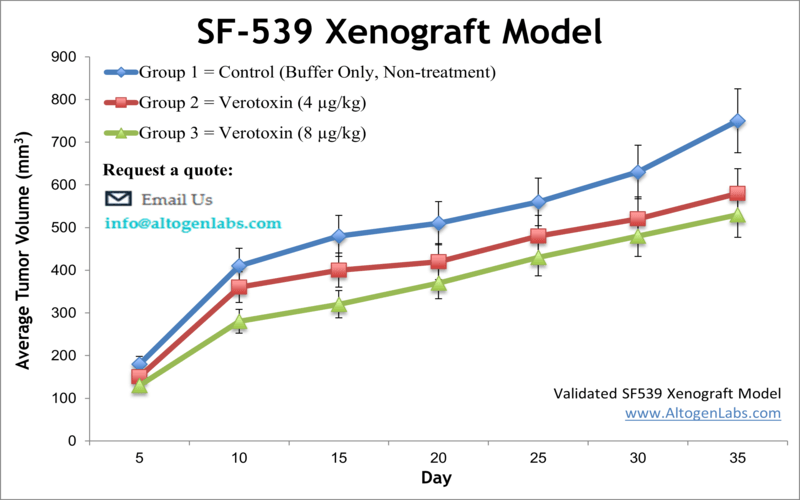
SF-539 xenograft model
SF539 is a human cancer cell line commonly used in research to study brain tumors. Gliosarcoma is a mixed tumor consisting of gliomatous and sarcomatous components, each of which can be diagnosed independently of the other. Malignant gliomas comprise approximately 35 to 45 percent of primary brain tumors and are characterized by uncontrolled cell proliferation within the brain and resistance to conventional treatment strategies due to obstacles in delivering drugs past the blood-brain barrier. Median survival after therapy is up to 10 months. The SF-539 cell line was isolated from a recurrent, right temporoparietal glioblastoma multiforme of a 34-year-old white female patient after the treatment. According to the study published in Cancer Research, the SF-539 cell line expresses collagen type IV, fibronectin, laminin, and procollagen type III. In 2013 Albanese et al. published a study using malignant glioma models, including SF-539, to demonstrate the ability of PHA-848125, a multi-kinase inhibitor, to cross the blood brain barrier and exhibit anti-tumor activity. This drug was proposed for phase 2 trials and its mechanism of action is reported to affect the cell cycle progression via cyclin-dependent kinase inhibition as well as pathways mediated by tyrosine kinase growth factors. SF-539 serves as a glioblastoma model in the National Cancer Institute NCI-60 cell panel. The SF-539 cell line is used to create the CDX (Cell Line Derived Xenograft) SF-539 xenograft mouse model. The SF-539 xenograft model enables a platform to test the anti-tumor efficacy of mono- and combination therapies (e.g. temozolomide, PHA-848125, radiotherapy).
SF539 Xenograft Model: Download ![]()
Download Altogen Labs SF-539 Xenograft Model PowerPoint Presentation: ![]()
Subcutaneous SF539 Xenografts in Preclinical Glioma Research
Subcutaneous xenograft transplantation is a widely utilized method in preclinical cancer research, valued for its technical simplicity, reproducibility, and capacity for real-time monitoring of tumor growth. In the case of the SF539 gliosarcoma cell line, subcutaneous implantation into immunocompromised mice has proven effective in establishing consistent and measurable tumor growth, making it a valuable model for evaluating therapeutic efficacy in vivo. Originating from a recurrent glioblastoma, SF539 cells exhibit distinct biological characteristics, including a mesenchymal phenotype and sensitivity to DNA-damaging agents such as temozolomide. Subcutaneous xenografts derived from SF539 cells offer a reliable system for studying drug response and tumor progression, particularly in early-stage screening of novel chemotherapeutics. Although this model lacks the native brain microenvironment and associated tumor–stroma interactions, it remains highly informative for assessing tumor kinetics and pharmacological response under controlled conditions. Recent studies have employed SF539 xenografts to explore the effects of targeted therapies, DNA repair inhibitors, and combination regimens, contributing to a growing body of literature that supports the model’s utility in translational neuro-oncology. Ongoing advancements in imaging technologies, molecular profiling, and co-engraftment strategies continue to improve the physiological relevance of subcutaneous xenografts, further reinforcing their role as a cornerstone of glioma drug development.
FKBP9 Drives Glioblastoma Progression and Stress Resistance
In a study published by the Journal of Experimental & Clinical Cancer Research, Xu H., et al. investigate the role of FKBP9 in glioblastoma (GBM) progression and resistance to endoplasmic reticulum (ER) stress. The authors demonstrate that FKBP9 is highly expressed in high-grade gliomas and correlates with poor patient prognosis across multiple datasets. Functional studies using GBM cell lines such as SF539, LN-229, and T98G revealed that FKBP9 knockdown significantly suppresses proliferation, invasion, and stemness, while also downregulating key survival and stem cell markers including Bcl-2, XIAP, Oct4, and Sox2. In vivo, FKBP9 depletion reduced tumor burden in both chick chorioallantoic membrane and mouse xenograft models. Mechanistically, the study identifies activation of the ASK1–p38MAPK pathway as a driver of FKBP9-mediated oncogenic activity. Additionally, FKBP9 suppresses the IRE1α-XBP1 branch of the unfolded protein response, conferring resistance to ER stress inducers such as tunicamycin and thapsigargin. FKBP9 itself is subject to proteasome-mediated degradation upon ER stress, with lysine 265 identified as a critical ubiquitination site.
The study’s methodology is comprehensive, integrating molecular and cellular assays with transcriptomic analysis and in vivo tumor models. The use of the SF539 glioma cell line, which exhibits mesenchymal characteristics, enhances the translational relevance of the findings. While the results strongly support FKBP9’s role as an oncogenic factor and regulator of ER stress tolerance, limitations include the use of immunodeficient animals, which precludes assessment of FKBP9’s immunological functions, and the absence of patient-derived xenograft validation. The research nonetheless contributes significantly to the field by identifying FKBP9 as a potential biomarker and therapeutic target in GBM. The findings suggest that targeting FKBP9 or its downstream signaling via the p38MAPK pathway, in combination with ER stress inducers, could improve treatment outcomes. Further studies are warranted to explore FKBP9’s interactome, its role across GBM subtypes, and its potential as a predictor of therapeutic response in clinical settings.
Pt2ad Shows Superior Cytotoxicity Over Cisplatin in SF539
A newly synthesized dinuclear organoplatinum(IV) complex stabilized by adenine, referred to as Pt2ad, has demonstrated potent cytotoxic activity in a subset of human cancer cell lines, with particularly striking results observed in the SF539 glioblastoma cell line. In vitro assays conducted using the sulforhodamine B method revealed that Pt2ad achieved a 50 percent growth inhibition (GI50) in SF539 at a concentration of 1.47 micromolar, total growth inhibition (TGI) at 2.76 micromolar, and a lethal concentration 50 (LC50) at just 5.20 micromolar. Notably, these values indicate a greater overall cytostatic and cytotoxic effect than either cisplatin or temozolomide, which are current standards in glioblastoma treatment but were shown to be ineffective against SF539 in this study. SF539, derived from a glioblastoma multiforme tumor, is known for its resistance to conventional chemotherapeutics, making this response to Pt2ad particularly significant. The selectivity of Pt2ad’s cytotoxicity, as demonstrated by its lack of effect on five other CNS cell lines, suggests a cell-specific mechanism that warrants further mechanistic investigation. These findings also reveal a disconnect between GI50 and LC50 values when comparing Pt2ad and cisplatin, highlighting a compound that is less immediately growth-inhibitory than cisplatin but more effective at halting cell division and inducing death over time. The experimental methodology is rigorous, leveraging the National Cancer Institute’s 60-cell line panel, but further studies are necessary to evaluate in vivo efficacy, biodistribution, and blood-brain barrier permeability. Early computational data suggest Pt2ad may penetrate the blood-brain barrier, though its high molecular weight and low water solubility may limit bioavailability. Further research should prioritize understanding the molecular underpinnings of SF539 sensitivity, potential platinum-DNA interactions, and structure-activity relationships, as well as assessing Pt2ad in orthotopic or patient-derived xenograft glioma models. These results position SF539 as a key model for evaluating the therapeutic potential of novel platinum-based compounds targeting glioblastoma.
Osimertinib Induces Paraptosis via ER Stress in Glioblastoma
The study by Hu et al., published in Cell Death Discovery journal, investigates the mechanism by which Osimertinib, a third-generation EGFR tyrosine kinase inhibitor, induces cell death in glioblastoma (GBM), with a particular focus on non-apoptotic pathways. Among the GBM cell lines used, SF539 plays a prominent role in confirming the unique cytotoxic effects of Osimertinib. The authors demonstrate that Osimertinib induces extensive cytoplasmic vacuolation and cell death in SF539, along with LN-229, U87MG, and LN-18 cells, through a mechanism independent of caspase activation and autophagy. This mode of death, identified as paraptosis, is strongly associated with endoplasmic reticulum (ER) stress and activation of the unfolded protein response, specifically the PERK–eIF2α–CHOP signaling pathway. In SF539 cells, Osimertinib treatment markedly increased the expression of CHOP and GRP78, as well as the accumulation of polyubiquitinated proteins, supporting the role of ER stress as a key mediator of cell death. RNA sequencing of Osimertinib-treated SF539 cells further confirmed the upregulation of ER stress-associated genes, reinforcing the centrality of the paraptotic response in this model. The authors employed a comprehensive methodological approach, including immunoblotting, electron microscopy, viability assays, and in vivo xenograft studies, to characterize the effects of Osimertinib. In SF539, both the genetic and pharmacological modulation of TRIP13, an AAA+ ATPase, significantly influenced the cell’s susceptibility to Osimertinib. TRIP13 overexpression in SF539 cells reduced ER stress marker expression and cytoplasmic vacuolization, thereby diminishing sensitivity to Osimertinib, while TRIP13 knockdown had the opposite effect. Importantly, treatment with the AKT inhibitor MK-2206 reduced TRIP13 levels and restored ER stress responses in SF539, enhancing the cytotoxicity of Osimertinib in vitro. These findings not only highlight SF539 as a representative and responsive glioma model but also underscore its utility in elucidating paraptotic mechanisms of cell death. While the study is limited by its use of immunocompromised animal models and requires clinical validation of TRIP13 as a biomarker, it introduces a promising strategy to overcome therapeutic resistance in GBM.
Structural Optimization and Selective Cytotoxicity in Glioma Cell Models
Hybrid compounds that combine Ciminalum and thiazolidinone scaffolds have shown strong anticancer activity, with notable effectiveness against central nervous system tumors. Among tested glioma models, the SF539 cell line demonstrated exceptional sensitivity, with significant growth inhibition and cytotoxicity observed at submicromolar concentrations. This high level of responsiveness suggests that SF539 is a particularly useful model for evaluating glioblastoma therapies involving small molecules that disrupt critical survival mechanisms in tumor cells. These compounds also showed selectivity, exhibiting minimal toxicity toward normal human lymphocytes, which indicates a potentially favorable therapeutic index. Structural variations on the thiazolidinone ring, especially at positions 3 and 5, had a substantial impact on biological activity. Substituents with different electronic properties altered the potency of the compounds, highlighting key structure activity relationships that can guide further optimization. While the data point to strong cytotoxic effects, the exact mechanisms by which these compounds act in SF539 cells remain undefined. Future investigations should determine whether the observed effects are driven by apoptosis, oxidative stress, or other forms of cell death. Moreover, linking the molecular profile of SF539, including DNA repair capacity, drug resistance markers, and metabolic traits, to its heightened drug sensitivity may reveal new therapeutic targets. These findings help expand our understanding of rational drug design for glioblastoma and support the development of targeted treatments for highly resistant brain tumors.
Oncogene Characteristics
Analysis of the SF539 glioblastoma cell line reveals that one of its principal oncogenic drivers is the MYC proto-oncogene, which is implicated in the regulation of mitochondrial DNA metabolism and transcription through its downstream effect on TOP1MT, a mitochondrial topoisomerase. According to Hu et al., SF539, along with other central nervous system (CNS)-derived tumor cell lines in the NCI-60 panel, exhibits low expression of TOP1MT. This mitochondrial enzyme plays a critical role in mtDNA relaxation and structural maintenance of mitochondrial nucleoids, and its transcription is directly regulated by MYC via E-box binding motifs in the TOP1MT promoter and first intron. The functional consequence of MYC-driven TOP1MT expression is the coordination of nuclear and mitochondrial gene expression to support bioenergetic demands and cell proliferation. However, in SF539 cells, both MYC and TOP1MT are expressed at relatively low levels compared to other cancer types, which may suggest a divergent metabolic profile or altered mitochondrial regulation in glioblastoma cells relative to more MYC-driven malignancies such as leukemias. Despite this low basal expression, the regulatory axis involving MYC and TOP1MT remains biologically relevant. MYC amplification or overexpression has been shown to increase TOP1MT levels and promote mitochondrial biogenesis, thereby enhancing oxidative phosphorylation and supporting rapid tumor growth. The absence of such amplification in SF539 suggests a potential vulnerability that could be exploited therapeutically, particularly in strategies targeting mitochondrial function or energy metabolism. Moreover, given MYC’s well-established role in driving cell cycle progression and evading apoptosis, its involvement in the modulation of mitochondrial gene networks positions it as a key integrator of metabolic and proliferative signals in glioblastoma. The unique oncogenic landscape of SF539, characterized by altered mitochondrial transcriptional regulation, supports the need for further research into how MYC and mitochondrial gene co-regulation influence glioma progression and therapeutic response. Future studies should investigate whether manipulation of the MYC–TOP1MT axis can sensitize SF539 cells to mitochondrial stress or targeted inhibitors, thereby opening new avenues for glioblastoma treatment
SF539 Xenograft Model: Download ![]()
Basic study design
- SF-539 cell culture is maintained under conditions of exponential growth.
- Each mouse receives a s.c. inoculation in the flank of one of the hind legs containing one million cells. The 100-150 microliter injection volume contains Matrigel plus SF-539 cells.
- The sites are palpated three times weekly till tumors are established.
- Tumors are measured (via digital calipers) until an average size of 100-200 mm3 followed by randomization and group assignment.
- Tumors measurements are taken on a daily basis, with accompanying body weights recorded 2 – 3 times a week.
- At the end of study, animals are euthanized humanely. Tissues are collected as defined for the termination of experiment. All tumors are excised and weighed; digital imaging is available.
- Tissues are collected for downstream analysis and are stabilized in RNAlater, snap frozen or prepared for pathology/histological analysis.
Get Instant Quote for
SF-539 Xenograft Model
Xenograft animal models are used to assess the effectiveness of test compounds against specific types of cancer. New medicines are tested on staged tumor growths that have been engrafted via subcutaneous or orthotopic inoculation in an immunocompromised mouse or rat model. All clinically approved anti-cancer agents have been evaluated with conventional preclinical in vivo models. Xenograft studies can be highly complex, starting with the selection of the appropriate animal model, choice of tumorigenic cell line, administration method, dosing, analysis of tumor growth rates and tumor analysis (pathology, mRNA and protein expression levels). Altogen Labs provides an array of laboratory services using over 90 standard Cell Line Derived Xenograft (CDX) models and over 30 PDX models. Researchers investigating the role of specific proteins or gene products in regulating tumor growth can benefit from development of protein overexpression (genetically engineered to ectopically express proteins, tumor suppressors, or oncogenes) and RNAi cell lines with long term gene silencing. Animal handling and maintenance at the Altogen Labs facilities are IACUC-regulated and GLP compliant. Following acclimation to the vivarium environment, mice are sorted according to body mass. The animals are examined daily for tumor appearance and clinical signs. We provide detailed experimental procedures, health reports and data (all-inclusive report is provided to the client that includes methods, results, discussion and raw data along with statistical analysis). Additional services available include collection of tissue, histology, isolation of total protein or RNA and analysis of gene expression.
Following options are available for the SF-539 xenograft model:
- SF-539 Tumor Growth Delay (TGD; latency)
- SF-539 Tumor Growth Inhibition (TGI)
- Dosing frequency and duration of dose administration
- SF-539 tumor immunohistochemistry
- Blood chemistry analysis
- Toxicity and survival (optional: performing a broad health observation program)
- Gross necropsies and histopathology
- Imaging studies: Fluorescence-based whole body imaging
