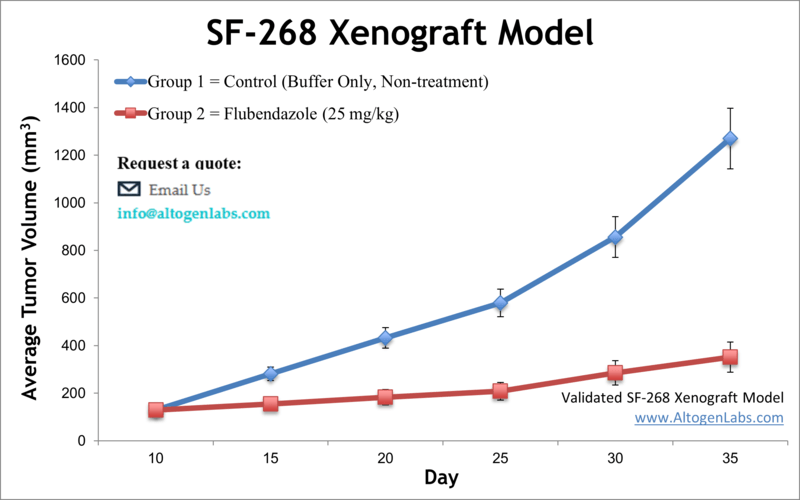
SF-268 xenograft model
SF268 cells were originally derived from a malignant glioblastoma tumor. According to the American Brain Tumor Association (ABTA), brain tumors are the most common pediatric malignancies and the primary cause of cancer fatalities in children. Approximately 80,000 new cases of brain tumors are detected annually among adults. Over one-third of them are malignant with 17,000 deaths per year. This cell line was thought to be established in 1987 from a female 24YO astrocytoma patient until further analysis revealed the presence of a Y chromosome. SF-268 is identified as being highly anaplastic, or lacking in distinct mature morphology, highly proliferative and likely aggressive. Many preclinical studies are carried out by using in vitro cellular models such as cancer cell lines that have proven to be essential model systems for exploring the fundamental properties of brain tumors. The SF-268 cell line (human brain; glioblastoma / astrocytoma) is used to create the CDX SF-268 xenograft mouse model. The SF-268 xenograft model exhibits overexpression of ID4, leading to enhanced angiogenesis, and is used in pre-clinical therapeutic agents targeting ID4. Among other studies using the SF-268 cell line is the 2018 Nature study by Zhou et al. which reported a novel utilization of the FDA approved drug Flubendazole for glioma treatment; the drug exhibited inhibition of cell proliferation, promotion of apoptosis as well as suppression of tumor growth in xenograft models through a cell cycle and apoptotic signaling mechanism of action. IN a study by Ashkenazi et al. published in the Journal of Clinical Investigation authors demonstrated Apo2L as a potential anticancer agent that induces apoptosis in tumor, but not normal cells. Previously, Westphal et al identified the SF268 cell line to show affinity to epidermal growth factor (EGF). In modern research, SF-268 is known as one of the brain tumor models used in the National Cancer Institute’s NCI-60 panel of cell lines used for standard screening of potential anticancer compounds program.
SF268 Brain Cancer Xenograft Model: Download ![]()
Download Altogen Labs SF-268 Xenograft Model PowerPoint Presentation: ![]()
At Altogen Labs, in preclinical studies, SF268 cells are cultured until they reach the exponential growth phase before being collected for injection. Viable cell counts are determined using trypan blue, and cell concentrations are quantified with a hemocytometer. The cell concentration is adjusted to ensure each mouse (athymic BALB/C or NOD/SCID, 10-12 weeks old) receives a subcutaneous injection in the hind leg, with 1 million SF-268 cells suspended in a 50% Matrigel solution for a 100 µL injection volume. In-study animals are monitored through palpation until tumor establishment is confirmed. Randomized treatment groups are formed based on an average tumor size of 100-150 mm³. The administration of test compounds is carried out in alignment with the treatment schedule, and tumor growth is tracked with daily measurements, alongside regular monitoring of the mouse’s body weight (three times weekly).
SF268 Glioma Model Reveals PI3K Inhibition as a Promising Therapeutic Strategy
Gliomas, aggressive primary brain tumors, often exhibit deregulation of the phosphatidylinositol 3-kinase (PI3K) signaling pathway, making it an attractive therapeutic target. The SF268 cell line, derived from human glioma, serves as a vital model system for studying the molecular effects of PI3K pathway inhibition. Using the selective PI3K inhibitor PI-103, researchers demonstrated potent suppression of PI3K pathway signaling in SF268 cells, evidenced by reduced phosphorylation of key proteins such as AKT, p70S6 kinase, and ribosomal protein S6. Notably, treatment with PI-103 induced a reversible cell cycle arrest at the G1 phase in SF268 cells, characterized by decreased expression of cyclin D1 and CDC6 and an increase in the cell cycle inhibitor p27Kip1. Interestingly, rather than triggering apoptosis, PI-103 induced significant autophagic responses, marked by extensive cytoplasmic vacuolation and LC-3 protein processing. Furthermore, when used in combination with common chemotherapy agents such as temozolomide, PI-103 displayed synergistic or additive effects, enhancing therapeutic outcomes in preclinical glioma models including SF268. These findings illustrate the potential of selective PI3K inhibitors to alter oncogenic signaling and improve therapeutic strategies in gliomas.
SF268 Cells Illuminate Potential of Neurological Therapeutics Against Glioma
Glioblastoma remains one of the most aggressive brain tumors, resistant to conventional therapies due to mechanisms such as the protective blood-brain barrier and intrinsic cellular resistance. To address this, existing drugs for neurological disorders capable of crossing the blood-brain barrier were evaluated for their potential antitumor effects using various neural tumor cell lines, notably including the SF268 glioma cell line. SF268 cells, characterized by resistance to temozolomide due to MGMT overexpression, served as a crucial model for studying novel drug activities. Among tested drugs, levomepromazine and fingolimod demonstrated significant antitumor effects on SF268 cells, notably reducing proliferation. Further assays showed that drugs such as levetiracetam, haloperidol, and valproic acid exhibited potent synergistic effects when combined with temozolomide, significantly enhancing anti-proliferative activity in SF268 cells. The cell death mechanisms identified included apoptosis induced by haloperidol, valproic acid, and levomepromazine, and autophagy induced by biperiden and dextromethorphan in SF268 cells. Additionally, fingolimod notably inhibited SF268 cell migration, a critical factor in glioma invasiveness. Collectively, these findings highlight SF268 as a valuable model for understanding resistance mechanisms and evaluating new therapeutic strategies in glioma treatment.
Get Instant Quote for
SF-268 Xenograft Model
Evaluating Glioblastoma Therapies with the SF-268 Subcutaneous Model
The subcutaneous SF-268 xenograft model is a well-established preclinical platform for studying glioblastoma and other central nervous system tumors. In this model, SF-268 cells are injected subcutaneously into immunodeficient mice, typically in the hind flank, allowing for controlled tumor growth monitoring. This model is widely used due to its reproducibility, ease of tumor measurement, and suitability for evaluating the efficacy of novel anticancer therapies. SF-268 tumors exhibit aggressive growth, making them ideal for assessing tumor progression, drug response, and resistance mechanisms. The subcutaneous model enables researchers to perform tumor growth inhibition (TGI) and tumor growth delay (TGD) studies, along with comprehensive pharmacokinetic and toxicity analyses. Additionally, tumor samples can be collected for histopathological evaluation, gene expression studies, and protein analysis to gain insights into tumor biology.
Flubendazole Suppresses Glioma Growth by Targeting SF-268 Cell Proliferation
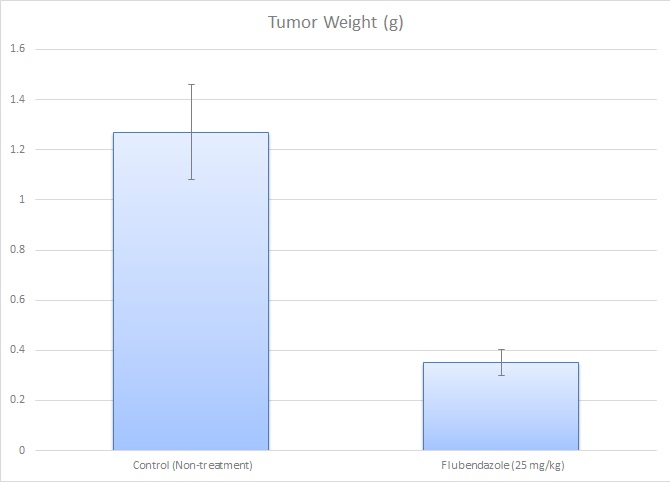
Flubendazole, an FDA-approved antiparasitic medication, exhibits notable anticancer effects against glioma, particularly through significant inhibition of proliferation and increased apoptosis. In a study by Zhou, X., et al., published by Cell Death Discovery journal, the glioma cell line SF-268 played a crucial role in demonstrating these effects, where flubendazole significantly inhibited its proliferation and clonogenic ability in vitro. In vivo experiments showed that flubendazole effectively suppressed tumor growth of SF-268 cells in xenograft models without affecting mice body weight or behavior, suggesting low toxicity. Notably, immunohistochemical analysis revealed reduced Ki-67 expression in SF-268 xenograft tumors, confirming diminished cellular proliferation. However, flubendazole did not significantly affect the migration capability or epithelial-mesenchymal transition markers in SF-268 cells. Mechanistic studies indicated that flubendazole triggered cell cycle arrest at the G2/M phase in SF-268 cells by increasing P53 expression while reducing cyclin B1 and p-cdc2 expression. Additionally, flubendazole induced apoptosis in SF-268 cells by downregulating anti-apoptotic Bcl-2 family proteins and upregulating cleaved caspases and PARP-1, reflecting activation of intrinsic apoptotic pathways. Collectively, these findings highlight SF-268 glioma cells as an important model demonstrating flubendazole’s potential as a novel therapeutic agent for glioma via its dual impact on cell cycle and apoptotic mechanisms.
SF268 Glioma Cells Illuminate the HOTTIP-miR-10b Axis in TMZ Resistance
This study explores the role of long non-coding RNA HOTTIP in the resistance of glioma cells to temozolomide (TMZ), a commonly used chemotherapy drug. Researchers utilized multiple glioma cell lines, notably highlighting SF268 due to its pronounced resistance to TMZ, making it an ideal model for investigating underlying resistance mechanisms. They identified significantly elevated expression of lncRNA HOTTIP in TMZ-resistant SF268 cells, indicating its critical role in resistance. The upregulation of HOTTIP was associated with increased proliferation, migration, angiogenesis, and metastatic markers in glioma cells. Mechanistically, SF268 cells displayed enhanced epithelial-to-mesenchymal transition (EMT), characterized by elevated ZEB1 and ZEB2 expression and decreased E-cadherin levels, mediated by increased miR-10b expression. Crucially, inhibition of miR-10b reversed EMT changes and resensitized SF268 cells to TMZ, confirming the functional link between HOTTIP, miR-10b, and TMZ resistance. These findings underscore the SF268 glioma cell line as a valuable model for studying the molecular mechanisms underpinning therapy resistance, particularly highlighting the HOTTIP-miR-10b-EMT signaling axis as a promising target for overcoming chemoresistance in glioma.
Chemotherapy: RES529 Enhances Glioma Therapy through TORC Inhibition
Glioblastoma (GBM) is an aggressive primary brain tumor characterized by poor prognosis and resistance to current therapies. Targeting key signaling pathways is crucial in developing new therapeutic strategies. The dual inhibitor RES529, which targets TORC1 and TORC2 signaling complexes, has shown promise in preclinical GBM models. Notably, RES529 significantly inhibited proliferation and tumor growth in GBM cell lines, particularly in the SF268 glioma model, which is often used as a representative glioma cell line. When combined with anti-angiogenic drugs like bevacizumab and sunitinib, RES529 enhanced therapeutic efficacy by delaying tumor recurrence and reducing angiogenesis and vasculogenic mimicry, two processes critical for glioma survival and invasion. Importantly, RES529 penetrates the blood-brain barrier, an essential property for treating brain tumors. Collectively, these findings suggest that RES529, especially when evaluated in the context of SF268 glioma cells, represents a promising approach to improving glioblastoma treatment outcomes.
Oncogenic YAP Activity and its Negative Regulation in SF-268 Cells
SF-268 is a glioma cell line frequently used as a model system to study oncogenic signaling in human cancers, particularly glioblastoma. A key feature of SF-268 cells is their elevated expression of the YAP oncogene due to genomic amplification, making them heavily reliant on YAP activity for proliferation and survival. YAP acts as a transcriptional co-activator downstream of the Hippo signaling pathway, promoting tumorigenesis when dysregulated. In SF-268 cells, high levels of YAP expression drive the activation of pro-growth and survival gene programs, directly contributing to cancer progression. The non-receptor tyrosine phosphatase PTPN14 is identified as a crucial negative regulator of YAP, binding directly and limiting its oncogenic activity by promoting cytoplasmic retention of YAP. Reduced expression of PTPN14 can mimic the effects of YAP activation, enhancing malignant transformation and survival under detached conditions (anoikis resistance), thus indicating its potential tumor-suppressive role. These interactions highlight a critical regulatory network within SF-268 cells, emphasizing the importance of the Hippo pathway and associated proteins like YAP and PTPN14 in glioma pathology.
The SF-268 cell line, derived from a central nervous system tumor, is widely utilized in preclinical brain cancer research due to its highly invasive nature and resistance to chemotherapy and radiation. The SF-268 xenograft model serves as a critical tool for studying tumor progression and therapeutic responses in a biologically relevant in vivo system. This model enables the investigation of tumor growth mechanisms, metastasis, and responses to various treatments, including chemotherapy, radiation, and immunotherapy. Altogen Labs offers multiple study options for the SF-268 xenograft model, including tumor growth delay (TGD) and tumor growth inhibition (TGI) studies, diverse dosing regimens and administration routes, immunohistochemistry, blood chemistry analysis, toxicity and survival assessments, and imaging studies such as fluorescence-based whole-body imaging.
Patient-Derived Organoids as Key Models in Precision Oncology
Organoids are three-dimensional (3D) culture systems that closely mimic the genetic and phenotypic complexity of patient-derived tumors. Unlike traditional two-dimensional cell cultures, organoids maintain the intricate tissue architecture and cellular heterogeneity found within original tumors, preserving genetic alterations, cellular differentiation patterns, and phenotypic diversity. Derived directly from primary patient samples, organoids offer a reliable and efficient approach for expanding tumor cells while maintaining their essential biological characteristics. This makes them particularly valuable in personalized oncology research, where treatment efficacy can vary significantly between individual tumors. While traditional xenograft and allograft models in animals offer important insights by incorporating tumor-stroma interactions and immune responses, organoids uniquely bridge the gap by enabling high-throughput and rapid assessment of therapeutic responses in vitro. Consequently, the establishment of patient-derived organoid libraries represents a major advancement, facilitating high-throughput drug screening and enabling precision medicine strategies tailored to individual patient tumor profiles. Their genetic stability, combined with ease of scalability and preservation of primary tumor heterogeneity, positions organoids as pivotal tools for the advancement of cancer therapeutics and personalized medicine strategies.
Basic study design:
- SF268 cells used for injection are grown at exponential growth phase until collected for injection. Viable cell counts are determined with trypan blue, and cell counts are determined by hemocytometer. Total cell concentration is adjusted such that each mouse (athymic BALB/C or NOD/SCID, 10-12 w.o.) receives a single injection (subcutaneous) in the hind leg. One million SF-268 cells in 50% Matrigel solution used for 100 µL injection volume.
- All in-study animals are monitored and continuously palpated until tumor establishment is determined. In-life study initiation begins as mice are sorted (randomization) into treatment groups with average tumor sizes of 100-150 mm3.
- Administrations of all test material compounds are performed in conjunction with the treatment schedule.
- Tumor growth is monitored by daily measurements, along with whole body mouse weight documentation (3 times weekly).
- End of study commences when tumor size reaches 2,000 mm3 (or determined size limit per approved IACUC protocol).
- Necropsies and tissue collections are performed. Tumors are excised and weighed (tumor imaging is available). Tissues are dissected into microtubes (snap frozen or immersed in RNAlater reagent), prepared for histology or nucleic acids isolated (RNA, small RNA, DNA, and/or protein fraction).
Xenograft models are generated by injecting either cells patient derived cells or established cell lines into immunodeficient rodents and exposing them to chemotherapy. This is one of the most reliable models that are used for cancer research. With improved, new technology, scientists have found different subgroups for the different types of brain tumors. Cell lines are the general term that applies to a defined population of cells that can be maintained in culture for an extended period. These are the types of cells that are permanently established and will reproduce quickly and indefinitely. Brain tumors are found to be one of the deadliest tumor and are most found in children. Xenograft models can also be obtained by primary tumors. These models called PDX have an enormous advantage of being directly generated from tumor cells, maintaining the features of the original neoplasm.
Animal handling and maintenance at the Altogen Labs facilities are IACUC-regulated and meet all GLP requirements. Following acclimation to the vivarium environment, mice are sorted according to body mass. The animals are examined daily for tumor appearance and clinical signs. We provide detailed experimental procedures, health reports and data (all-inclusive report is provided to the client that includes methods, results, discussion and raw data along with statistical analysis).
SF268 is a human cancer cell line that was derived from a central nervous system tumor and is commonly used in preclinical brain cancer research. SF268 cells have been extensively characterized and are known to be highly invasive and to exhibit resistance to chemotherapy and radiation therapy, properties that are typical of aggressive brain tumors. SF268 xenograft model is a powerful research tool in brain cancer research because they allow for the study of tumor growth and response to treatment in a living system that more closely resembles the human body than in vitro / cell culture studies. SF268 xenograft model is used to study the biology of brain tumors, including the mechanisms of tumor growth and metastasis, as well as the tumor’s response to various treatments such as chemotherapy, radiation therapy, and immunotherapy.
Following options are available for the SF-268 xenograft model:
- SF-268 Tumor Growth Delay (TGD; latency)
- SF-268 Tumor Growth Inhibition (TGI)
- Dosing frequency and duration of dose administration
- Dosing route (intravenous, intratracheal, continuous infusion, intraperitoneal, intratumoral, oral gavage, topical, intramuscular, subcutaneous, intranasal, using cutting-edge micro-injection techniques and pump-controlled IV injection)
- SF-268 tumor immunohistochemistry
- Alternative cell engraftment sites (tail vein injection and left ventricular injection for metastasis studies, injection into the mammary fat pad, intraperitoneal injection)
- Blood chemistry analysis
- Toxicity and survival (optional: performing a broad health observation program)
- Gross necropsies and histopathology
