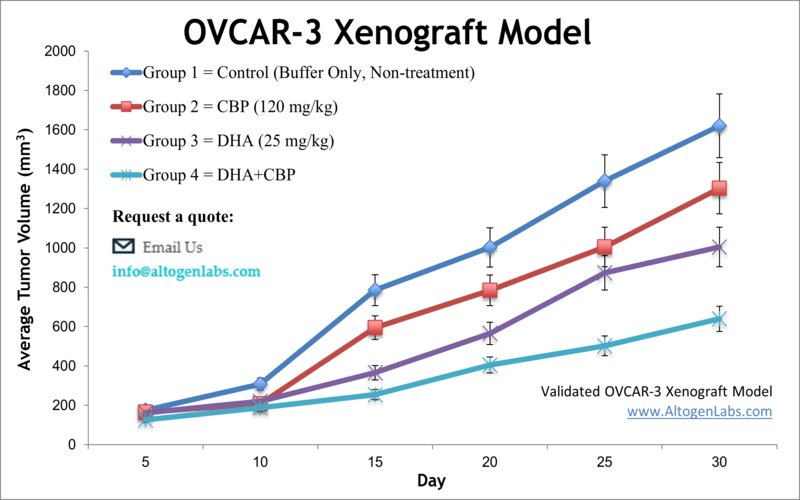
OVCAR-3 xenograft model
OVCAR-3 cells are known to express high levels of the estrogen receptor (ER) and progesterone receptor (PR), and they are sensitive to hormones such as estradiol and progesterone. They also express high levels of the cancer antigen 125 (CA-125), which is a marker for ovarian cancer. Ovarian cancer is the fifth-primary cause of cancer deaths among women, accounting for more fatalities than any other type of cancer in females. Ovarian cancer mostly occurs in older Caucasian women over 60 years old and is one of the deadliest gynecological malignancy worldwide, as indicated by the American Cancer Society. The OVCAR3 cell line was isolated by Hamilton et al. from the malignant ascites of a patient with progressive adenocarcinoma of the ovary. These cells were characterized in a xenograft model by Hamilton et al. in Cancer Research in 1984, where data demonstrated that mice developed a similar metastatic spread to clinical ovarian cancer and that tumors manifest cytoplasmic estrogen and androgen receptors as well as CA125, the ovarian cancer associated antigen. Similarly, a 2015 study in Gynecology Oncology (Mitra et al.) looked at several frequently used ovarian cancer models, including OVCAR-3, in order to evaluate which cell lines are most reliable for cancer studies. Results looked at tumor formation, ascite formation, histology and immunohistochemistry of PAX8, p53 and WT1 expression and authors used this data to define which models can best represent high-grade serous ovarian cancer (HGSOC). A 2016 study by Park et al. published in Cancer Investigation examined the tumor-suppressive properties of enzalutamide on androgen-driven ovarian cancer in vivo using the OVCAR-3 xenograft model; combination treatment of dihydrotestosterone with enzalutamide led to significant reductions in tumor volume in the OVCAR3 xenograft model in vivo. These findings indicate that the second-generation antiandrogen enzalutamide could be effective in the treatment of ovarian cancer. The OVCAR3 cell line is used to create xenograft mouse model, that is utilized to monitor in vivo tumor growth suppression by alternative therapeutic strategies (e.g. vitamin supplementation EB1089), gene silencing (e.g. claudin-3 siRNA) or small molecules targeting early relapse due to lack of HER2 amplification (e.g. trastuzumab).
Download Altogen Labs OVCAR3 Xenograft Model PowerPoint Presentation: ![]()
Basic study design
- Cells are maintained within a phase of exponential growth and then collected via trypsinization. Cell viability (trypan blue assay) and cell count (flow cytometry / hemacytometer) is determined; cell count of the suspension is adjusted to 10,000 cells/µL. The Matrigel + OVCAR3 cell suspension is injected (100-150 µL) into the rear flank of athymic BALB/C mice (11 to 12 weeks old).
- As tumors reach 75-125 mm3, mice are sorted into treatment cohorts (randomization). All test compounds are administered in agreement with the study design treatment schedule.
- Tumor measurements (daily) are logged. Whole body weights are determined up to 2-3 times a week.
- The in-life portion of the study ends when the study design tumor size limit is reached (or there is animal health concerns) per approved IACUC protocol tumor size. Humane euthanization enables tissues/tumors to be collected.
- All removed tumors are weighed and documented. Additionally, tumors can be digitally imaged.
- Tissues collected for downstream analysis are stabilized by snap freezing, stabilization in RNA Later reagent, submersed in 10% NBF formalin, or isolation of nucleic acids.
Get Instant Quote for
OVCAR-3 Xenograft Model
Xenograft animal models are used to assess the effectiveness of drugs against specific types of cancer. New medicines are tested on staged tumor growths that have been engrafted via subcutaneous or orthotopic inoculation in an immunocompromised mouse or rat model. All clinically approved anti-cancer agents have been evaluated with conventional nonclinical in vivo models. Xenograft studies can be highly complex, starting with the selection of the appropriate animal model, choice of tumorigenic cell line, administration method, dosing, analysis of tumor growth rates and tumor analysis (histology, mRNA and protein expression levels). Altogen Labs provides an array of laboratory services including safety toxicology and efficacy xenograft studies. Researchers investigating the role of specific proteins or gene products in regulating tumor growth can benefit from development of protein overexpression (genetically engineered to ectopically express proteins, tumor suppressors, or oncogenes) and RNAi cell lines with long term gene silencing. Altogen Labs provides quantitative gene expression analysis of mRNA expression (qPCR) and protein expression analysis using automated western blot WES system. Animal handling at the Altogen Labs facility is IACUC regulated and GLP compliant. Following acclimation to the vivarium environment, mice are sorted according to body mass. The animals are examined daily for tumor appearance and clinical signs. We provide detailed experimental procedures, health reports and data (all-inclusive report is provided to the client that includes methods, results, discussion and raw data along with statistical analysis).
Following options are available for the OVCAR-3 xenograft model:
- OVCAR-3 Tumor Growth Delay (TGD; latency)
- OVCAR-3 Tumor Growth Inhibition (TGI)
- Dosing frequency and duration of dose administration
- Dosing route (intravenous, intratracheal, continuous infusion, intraperitoneal, intratumoral, oral gavage, topical, intramuscular, subcutaneous, intranasal, using cutting-edge micro-injection techniques and pump-controlled IV injection)
- OVCAR-3 tumor immunohistochemistry
- Alternative cell engraftment sites (orthotopic transplantation, tail vein injection and left ventricular injection for metastasis studies, injection into the mammary fat pad, intraperitoneal injection)
- Blood chemistry analysis
- Toxicity and survival
- Gross necropsies and histopathology
- Imaging studies: Fluorescence-based whole body imaging
