HT29 xenograft model (subcutaneous and metastatic)
HT-29 is a human colon cancer cell line that was originally derived from a primary adenocarcinoma tumor in a patient with colon cancer. It is widely used in cancer research as a model system to study various aspects of cancer biology, including cell proliferation, differentiation, and drug resistance. Colon cancer represents the third most prevalent malignancy among both males and females and the second primary cause of cancer-related deaths in the United States. HT29 is a human colorectal adenocarcinoma cell line that was derived from a tumor of a 44-year-old Caucasian woman in 1964 and is helpful in gaining insight into the etiology of human colon cancers. The HT29 colorectal cell line is vital for examining biochemical pathways that aid in the development of colon carcinoma. Many colorectal rodent models have been developed to facilitate studies of human malignancies, allowing a better understanding of mechanisms contributing to tumor growth. Example studies using the HT29 model include the Cancer Research article by Radulovic et al. in which the group identified that RC-3095, a peptide agonist designed to release bombesin and gastrin, successfully inhibited tumor growth in HT29 xenografts. This study contributed to knowledge regarding involvement of sex steroids, hormones and growth factors on colorectal tumorigenesis. HT29 was featured in a model spotlight in 2016 (Maryland Franklin, PhD, MI Bioesearch) due to its high utilization and success in helping identify chemotherapy treatments. A 2016 Nature study (Teng et al.) used HT29 as a CRC model to demonstrate the oncogenic role of protein tyrosine phosphatase 1B (PTP1B); results confirmed that PTP1b predicts poor clinical outcomes in patients, identified PITX1 as a novel substrate on the p120RasGAP axis and found that the drug regorafenab has an antitumor effect that is mediated by PTP1B. This study established a role of PTP1B in colorectal cancer and demonstrated its potential as a biomarker for predicting the effectiveness of regorafenib. Finally, a 2014 study in Oncoscience (Gotnik et al.) used the HT29 model to study the mechanism of resistance of colorectal cancer against sunitinib, a tyrosine kinase and angiogenesis inhibitor. Often tumor resistance can be attributed to microenvironmental host-factors such as tumor microvasculature or biodistribution however results showed that resistant tumor cells demonstrated increased sequestration in cells via lysosomes. Therefore the study concluded to overcome sinitinib resistance, combination therapy with a treatment that inhibits lysosomal function may be beneficial. The HT29 cell line is used to create the CDX (Cell Line Derived Xenograft) HT29 xenograft mouse model. The HT29 xenograft model enables analysis of the anti-tumor efficacy of EGFR inhibitors (e.g. cetuximab) and to elucidate the mechanism of acquired resistance to tyrosine kinase inhibitors (TKIs), such as using a sunitinib-resistant HT29 cell line.
HT-29 Colorectal Cancer Xenograft Model: Download ![]()
Download Altogen Labs HT29 Xenograft Model PowerPoint Presentation: ![]()
Subcutaneous Transplantation Models Using HT29 Cells
Subcutaneous xenograft transplantation is a foundational technique in preclinical oncology, offering a reliable platform to study tumor growth and therapeutic efficacy in vivo. The HT29 colorectal adenocarcinoma cell line, derived from a human primary tumor, is a well-established model in this context due to its stable genetic background, reproducible tumorigenicity, and relevance to the serrated pathway of colorectal carcinogenesis. HT29 cells carry mutations in APC, TP53, and BRAF (V600E) and can form moderately differentiated tumors when injected subcutaneously into immunodeficient mice, such as NOD/SCID or athymic nude strains. These xenografts exhibit consistent growth kinetics and histopathological characteristics, making them ideal for evaluating chemotherapeutics, investigating drug resistance, and analyzing molecular pathways involved in colorectal tumor progression.
While subcutaneous models lack the native tumor microenvironment and immune system interactions present in orthotopic or syngeneic models, they remain valuable for controlled studies of tumor biology. HT29 xenografts have been used to assess responses to standard treatments such as 5-fluorouracil and irinotecan, as well as to explore novel therapeutics including nanoparticle-based drug delivery systems. Integration of this model with histological, proteomic, and transcriptomic analyses enables mechanistic insights into treatment response, angiogenesis, and tumor cell signaling. Although limitations persist, emerging strategies such as the incorporation of humanized mice or co-engraftment with stromal or immune cells may further enhance the translational relevance of HT29 subcutaneous xenografts in colorectal cancer research.
Orthotopic Modeling of HT29 Colorectal Tumors
Orthotopic xenograft transplantation has emerged as a critical model for investigating the progression and therapeutic response of colorectal cancer in a context that closely reflects the anatomical and physiological environment of the human colon. The HT29 colorectal adenocarcinoma cell line, widely used for its stable genetic profile and reproducible tumorigenic properties, has been effectively employed in orthotopic transplantation studies to mimic the native tumor microenvironment. By implanting HT29 cells directly into the cecal wall or rectum of immunodeficient mice, researchers can replicate the spatial and biological conditions that drive tumor growth, local invasion, and distant metastasis in human colorectal cancer.
Orthotopic HT29 models offer significant advantages over traditional subcutaneous systems by preserving the complex interactions between tumor cells and surrounding stromal, vascular, and epithelial components. These interactions are essential for accurately studying key aspects of tumor biology, including local invasion, angiogenesis, and metastatic potential. Furthermore, orthotopic transplantation facilitates the emergence of spontaneous metastases, thereby providing a valuable framework for assessing the efficacy of systemic therapies in a clinically relevant setting. While the absence of a functional immune system in the host remains a limitation, orthotopic HT29 xenografts continue to advance our understanding of colorectal tumor behavior within a native microenvironment, bridging the gap between preclinical experimentation and translational cancer research.
Dual Inhibition of MEK and EGFR Overcomes Resistance in HT29 Cells
HT29 colorectal cancer cells, which harbor a BRAF V600E mutation, display limited responsiveness to MEK inhibition due to feedback reactivation of signaling pathways. Treatment with the MEK inhibitor AZD6244 suppresses MAPK signaling and partially inhibits proliferation; however, this effect is transient. Over time, levels of phosphorylated ERK (p-ERK) and AKT (p-AKT) rebound, accompanied by upregulation of HER3, a member of the EGFR family. These compensatory changes diminish the long-term efficacy of AZD6244 alone. The addition of cetuximab, an EGFR-targeting monoclonal antibody, significantly enhances the antitumor activity of AZD6244. In HT29 cells, this combination reduces the AZD6244 IC50 five-fold, induces more pronounced G1-phase cell cycle arrest, and significantly increases apoptosis, as evidenced by elevated cleaved PARP and Annexin V staining. The combination also prevents HER3- and AKT-mediated feedback activation, sustaining inhibition of both MAPK and PI3K signaling pathways. This synergistic interaction is further supported by in vivo xenograft data. In nude mice bearing HT29-derived tumors, combination therapy with cetuximab and AZD6244 results in significantly smaller tumor volumes compared to monotherapy or control groups. Histological analysis of excised tumors reveals reduced expression of HER3 and p-AKT, confirming molecular effects observed on vitro. These findings highlight the importance of dual-pathway targeting to overcome adaptive resistance mechanisms in BRAF-mutant colorectal cancers. While cetuximab is typically ineffective in such contexts when used alone, its ability to disrupt HER3-mediated feedback may explain the improved outcomes when paired with MEK inhibition. This approach underscores the potential of rationally designed combination therapies to enhance treatment efficacy in molecularly stratified colorectal cancers. Further investigation is warranted to determine whether similar effects are observed in other BRAF-mutant tumor models and to explore additional feedback loops that may limit therapeutic durability.
Redundant Oncogene Signaling Drives HT29 Tumor Growth
The HT29 colorectal cancer model illustrates how key oncogenes such as MYC, mutant KRAS, and mutant TP53 contribute to tumorigenesis not through cooperative enhancement but through competitive and functionally redundant mechanisms. In HT29 cells, which harbor all three oncogenic drivers, transcriptomic and proteomic profiling following CRISPR-Cas9-mediated downregulation of each gene revealed that these oncogenes activate many of the same downstream targets independently. Critical effector genes including RUVBL1, HSPA9, and XPO1 were regulated by each oncogene, but co-expression did not amplify their activity. Instead, one oncogene often assumed dominance in controlling these targets while suppressing the influence of the others. Chromatin immunoprecipitation and transcription factor dependency analyses showed that MYC, KRAS, and mutant TP53 bind competitively to gene promoters and signal through distinct regulatory pathways, including c-Jun, GLI2, NFYA, and NFKB1.
Modeling Metastasis with HT29 Xenografts
Metastatic xenograft transplantation models are essential for investigating the mechanisms underlying colorectal cancer dissemination and for evaluating therapeutic strategies targeting metastatic disease. The HT29 colorectal adenocarcinoma cell line, characterized by its epithelial morphology and well-defined mutational profile, has been effectively utilized in the development of metastatic xenograft models. These models enable the study of tumor cell colonization in distant organs, most notably the liver and lungs, which represent common sites of metastasis in colorectal cancer patients. Unlike subcutaneous models, metastatic xenografts more closely mimic the clinical behavior of advanced-stage tumors and provide a dynamic platform for assessing the efficacy of anti-metastatic interventions.
HT29 cells can be introduced into mice using routes that facilitate metastatic spread, such as intrasplenic, tail vein, or orthotopic implantation. These methods support the establishment of spontaneous or experimental metastases and allow for the observation of tumor progression within organ-specific microenvironments. Tumors derived from HT29 cells in these models often display distinct molecular adaptations associated with increased invasiveness and survival in secondary tissues. The metastatic HT29 xenograft system is particularly valuable for examining alterations in cell adhesion, migration, angiogenesis, and immune evasion during the metastatic process.
Basic study design
- Prior to collecting the cells, exponential growth is maintained.
- All cells are collected by trypsinizing the cells in flasks and combining all cell suspensions. Cell count and viability is properly determined using trypan blue (min 99% viability). Cell suspensions are adjusted to an appropriate density for inoculation.
- One million cells of the Matrigel-HT29 cell suspension (vol=100 µL) is injected s.c. into the flank of one hind leg of each mouse (10 to 12 weeks; NOD/SCID or athymic BALB/C).
- All injection sites are monitored until tumors (palpated) are established. Calipers (digital) are used to measure the tumors pending average sizes of 80-120 mm3.
- After sorting (randomization), the test materials are administered according to the client supplied treatment schedule.
- Daily measurements of the tumor are logged and mouse weights are recorded (up to 3 times weekly).
- The animals are euthanized humanely as the study’s maximum tumor size is reached (or 2,000 mm3).
- A necropsy is performed to remove the tumors, record their weight and then document with digital imaging.
- Tissues are collected as discussed and are stabilized in RNAlater, snap frozen, nucleic acids isolated or tissues are prepared for histological analysis.
Get Instant Quote for
HT29 Xenograft Model
HT-29 Colorectal Cancer Xenograft Model: Download ![]()
Metastatic Model
CDX models are mouse xenografts used in pre-clinical therapeutic studies. However, as primary tumors proliferate they invade surrounding tissue, become circulatory, survive in circulation, implant in foreign parenchyma and proliferate in the distant tissue. This result leads to an extremely high percentage of death in cancer patients due to metastasis. Metastatic tumor mouse models are utilized to develop novel therapeutic agents that target metastasis (anti-metastatic therapeutics). To create a metastatic model, the cell line of interest is transfected with vectors containing green fluorescent protein (GFP) or luciferase. Maintained under antibiotic selection, only cells containing the integrated vector will survive. The new cell line clones are capable of stably expressing the gene of interest and are used in metastatic mouse model studies. Although each new cell line clone may contain its own inherent difficulties, the new cell line contains the ability to track internal tumor progression via bioluminescence (luciferase fluorescence after injecting luciferin) or fluorescence (GFP). Internal orthotopic and metastatic tumor growth (not palpable) can now be measured throughout the study, enabling a researcher to gain more insight and additional data in contrast to relying on end of study tumor weight measurements.
Case Study: U87-luc Xenograft Model
An example of Altogen Labs utilizing a luciferase expressing cell line to monitor orthotopic tumor growth is exhibited below. The same ideology of tumor observation is incorporated in metastatic tumor models. Luciferase expressing U87-luc cells were implanted and tumors allowed to grow. Tumor growth was monitored in a Night Owl (Berthold Technologies) imaging system 10 minutes after an intraperitoneal (IP) injection of the luciferin substrate. As seen in the example below, luciferase expression (measured as photons emitted) in the U87-luc model grants the researcher a visual image and quantifiable metric for orthotopic or metastatic tumor progression.
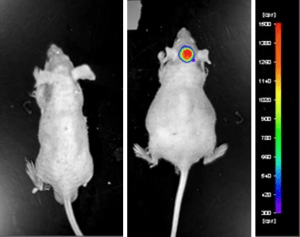
Figure 1. Luciferase expression in U87-luc orthotopic model. Control and implanted glioma mouse model fluorescence was analyzed 10 minutes after intraperitoneal luciferin injection.
View full details of the case study by clicking here.
