NCI-N87 xenograft model
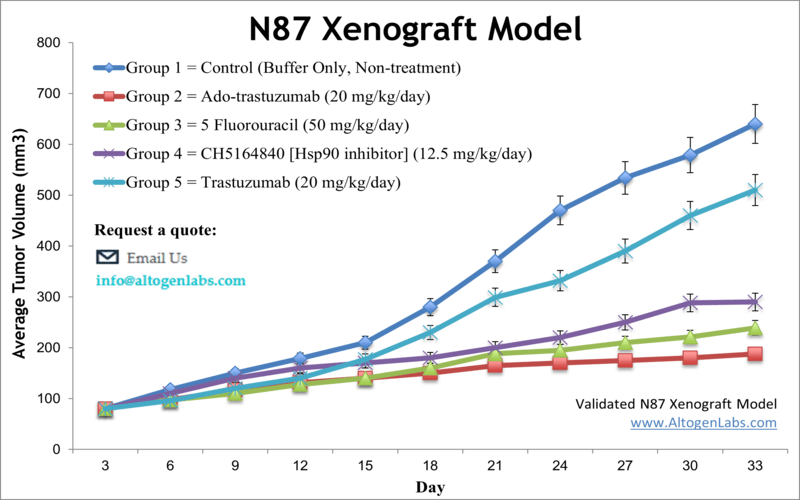
NCI-N87 is a human gastric adenocarcinoma cell line that was originally derived from a patient with gastric adenocarcinoma.Gastric cancer is one of the most prevalent malignancy worldwide. Gastric adenocarcinomas are typically associated with high mortality and limited treatment options. Subcutaneous implantation of the NCI-N87 human gastric tumor cells into immunocompromised mice can be utilized as a platform to test new drug candidates as well as predict in vivo drug responses in humans. Xenograft murine models aid in the investigation of targeted agents aiming to improve the clinical outcome of gastric cancer patients. The NCI-N87 tumorigenic epithelial cell line was derived from a metastatic site in the liver of a male patient with gastric carcinoma. NCI-N87 has been positively tested for myc and erb B2 oncogenes. Furthermore, it expresses carcinoembryonic antigen as well as TAG 72 surface glycoproteins. The NCI-N87 cell line is EGFR-positive, human epidermal growth factor receptor 2 (HER2) positive and responds to cetuximab as well as carboplatin both in vitro and in vivo, as per a 2012 study in Gastric Research. The 2015 Molecular Clinical Oncology study (Harada et al.) also used the NCI-N87 model to test combination therapy in HER2+ human gastrinoma. A common combination chemotherapy regiman include capecitabine/oxaliplatin (XELOX), 5-fluorouracin (5-FU)/cisplatin, or trastuzumab/capecitabine/cisplatin and this study identified that trastuzumab with XELOX resulted in upregulation of thymidine phosphorylase (TP) and may mediate interactions between NK and NCI-N87 cells; this has potential for clinical translation. Xhang et al. (2013) also used the NCI-N87 cell line to successfully establish a peritoneal gastric carcinoma xenograft model to support survival outcome analysis. The last example study is the Clinical Cancer Research article by Yamashita-Kashima et al. (2011) which used the NCI-N87 model to demonstrate the significant efficacy enhancement of combination therapy with pertuzumab and trastuzumab against HER2+ gastric carcinoma; the mechanism of action was shown to inhibit cell growth, increase apoptosis and ADCC-mediated cell killing as well as have antiangiogenic activity. These cells also have a mutation in the TP53 gene, which encodes for the p53 tumor suppressor protein. The NCI-N87 cell line is used to create the xenograft mouse model. NCI-N87 is a HER2-positive gastric cancer xenograft model utilized in studying pre-clinical monotherapies and combination therapies (e.g. pertuzumab, trastuzumab, 5-FU). NCI-N87 cells are characterized by their high expression of the human epidermal growth factor receptor 2 (HER2), which is a protein that is overexpressed in a subset of gastric cancers and is associated with poor prognosis.
Download Altogen Labs NCI-N87 Xenograft Model PowerPoint Presentation: ![]()
Basic study design
- After continuous cell growth at exponential phase, NCI-N87 cells are trypsinized, viability and cell count determined and suspension concentration adjusted prior to injection.
- 1 x 106 cells (vol = 100 µL) of a Matrigel + NCI-N87 suspension are injected in 12 week old athymic BALB/C (nu/nu) mice. Cells are subcutaneously injected to the rear hind leg (flank). Injection sites are inspected and palpated until tumors are established.
- Calipering of tumors allows the in-life portion of the study to begin when an average size of 50-150 mm3is reached. Randomization, based on tumor measurements, into client determined treatment groupings is performed. Injections of the client supplied test material follows the treatment schedule. Daily tumor measurements (digital calipers) and mouse whole body weights (3 times weekly) are recorded.
- Animals are sacrificed and tissues are collected. As instructed by client, tissues can be frozen (LN2), stabilized (RNAlater) or fixed for histological analysis (10% NBF). Additionally, tumors are weighed and documented (digital imaging).
Get Instant Quote for
NCI-N87 Xenograft Model
Altogen Labs provides an array of laboratory services using over 90 standard Cell Line Derived Xenograft (CDX) models and 30+ PDX models. Researchers investigating the role of specific proteins or gene products in regulating tumor growth can benefit from development of protein overexpression (genetically engineered to ectopically express proteins, tumor suppressors, or oncogenes) and RNAi cell lines with long term gene silencing. Altogen Labs provides quantitative gene expression analysis of mRNA expression (RT-PCR) and protein expression analysis using the WES system (ProteinSimple).
The dosing of the experimental compound of interest is initiated, for a staged study, when the mean tumor size reaches a specified volume (typically 50-100 mm3). In an unstaged study, the dosing of the compound of interest is initiated immediately after xenografting. Mice are dosed once or twice a day for 28 days (or other desired study duration) via the chosen route of administration. Tumor volume (mm3) is calculated via the “(W x W x L) / 2” formula, where W is tumor width and L is tumor length.
Animal handling and maintenance at the Altogen Labs facility is IACUC-regulated and GLP-compliant. Following acclimation to the vivarium environment, mice are sorted according to body mass. The animals are examined daily for tumor appearance and clinical signs. We provide detailed experimental procedures, health reports and data (all-inclusive report is provided to the client that includes methods, results, discussion and raw data along with statistical analysis). Additional services available include collection of tissue, histology, isolation of total protein or RNA and analysis of gene expression. Our animal facilities have the flexibility to use specialized food or water systems for inducible gene expression systems.
Following options are available for the NCI-N87 xenograft model:
- NCI-N87 Tumor Growth Delay (TGD; latency)
- NCI-N87 Tumor Growth Inhibition (TGI)
- Dosing frequency and duration of dose administration
- Dosing route (intravenous, intratracheal, continuous infusion, intraperitoneal, intratumoral, oral gavage, topical, intramuscular, subcutaneous, intranasal, using cutting-edge micro-injection techniques and pump-controlled IV injection)
- NCI-N87 tumor immunohistochemistry
- Alternative cell engraftment sites (orthotopic transplantation, tail vein injection and left ventricular injection for metastasis studies, injection into the mammary fat pad, intraperitoneal injection)
- Blood chemistry analysis
- Toxicity and survival (optional: performing a broad health observation program)
- Gross necropsies and histopathology
- Positive control group employing cyclophosphamide, at a dosage of 50 mg/kg administered by intramuscular injection to the control group daily for the study duration
- Imaging studies: Fluorescence-based whole body imaging, MRI
