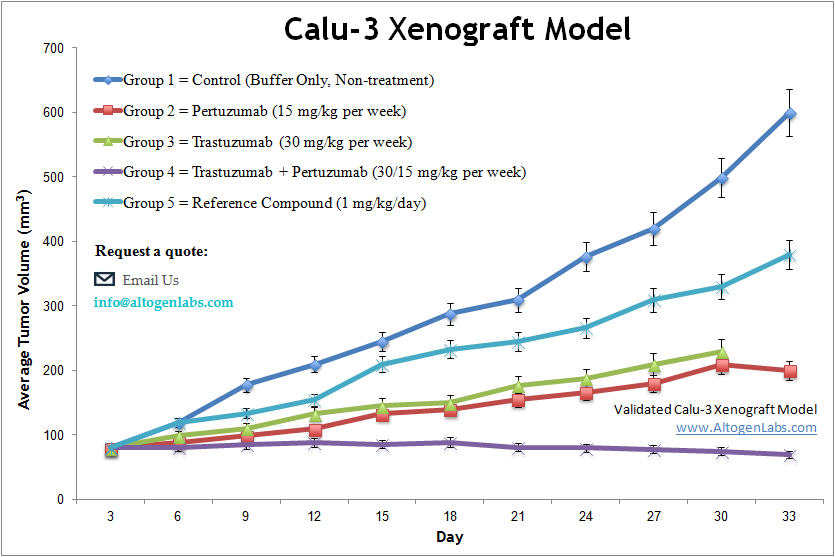
Calu3 xenograft model
Non-small cell lung cancer (NSCLC) is the leading cause of cancer-related death worldwide and accounts for roughly 85 percent of lung cancer cases. Tyrosine kinase (TK) receptors, including vascular endothelial growth factor (VEGF) receptor (VEGFR), play a critical role in tumor angiogenesis. Inhibition of TK receptors is a promising approach for cancer treatment. The tumorigenic epithelial Calu-3 cell line is derived initially from the lung tissue of a 25-year-old Caucasian male with adenocarcinoma. In 2015 Kreft et al. released a study characterizing the Calu-3 cell line culture conditions and highlighted the different effects varied environments take on Calu-3 behavior. They showed that properties that are affected by culture conditions include permeability properties, barrier integrity, cell differentiation and drug transporter expression which are important with studies for drug screening. Another 2015 study published in Lung Cancer evaluated acute vascular response to cediranib in the Calu-3 NSCLC xenograft model. The article indicates that the perfusion of Calu-3 tumors was significantly reduced twenty-four hours after cediranib administration, with a significant increase in hypoxia. Therefore, tumor stromal architecture may be associated with acute tumor vascular response to VEGFR TKI that could be an early predictive marker of response to VEGFR TKI in NSCLC. A 2001 study by Stein et al. used the Calu-3 xenograft model to test the efficacy of a combination of radioimmunotherapy with the monoclonal antibody RS7, an antiepithelial glycoprotein 1 agonist. The antibody was labeled with (177)-Lu and results suggested that this (177)Lu-RS7 is a viable radioimmunoconjugate for radioimmunotherapy as xenografts demonstrated growth inhibition and tumor regression. Lastly, the Oncology Letters study by Yang et al. used Calu-3 xenografted mice to test the antitumor properties of polysaccharides from Scutellaria barbata D. Don (PSB), a Lamiaceae family perennial herb that is a traditional Chinese medicine used as an anti-inflammatory and anti-tumor agent. Data demonstrated treatment resulted in inhibition of angiogenesis via the human epidermal growth factor receptor (HER) 2 pathway, establishing PSB as a potential anti-cancer drug therapy. The Calu-3 cell line (human lung) is used to create the CDX (Cell Line Derived Xenograft) Calu-3 xenograft mouse model. The Calu-3 tumor model is a well-established xenograft model to study the effects therapeutics, such as erlotinib, trastuzumab and pertuzumab on HER2 and EGFR expressing cells. Calu-3 xenografts are commonly used to investigate the effects of various drugs and compounds on tumor growth and metastasis. They are also useful for studying the molecular mechanisms of lung cancer progression and drug resistance in vivo, and for identifying potential targets for the treatment of lung cancer.
Download Altogen Labs Calu3 Xenograft Model PowerPoint Presentation: ![]()
Calu-3 Subcutaneous Tumor Model: Download ![]()
Calu-3 non-small cell lung cancer (NSCLC) cells demonstrate distinct oncogenic behavior primarily through ERBB2 (HER2) gene amplification, resulting in hyperactivation of the PI3K/AKT/mTOR signaling axis. This signaling promotes uncontrolled cellular proliferation and survival, contributing to tumor progression and therapeutic resistance. Notably, Calu-3 cells exhibit pronounced sensitivity to epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs), such as erlotinib, due to their specific oncogenic landscape. These molecular alterations position Calu-3 as a critical model for investigating targeted therapeutic strategies against ERBB2-driven lung carcinogenesis. A comprehensive understanding of Calu-3’s oncogenic signaling networks may facilitate the development of precision therapies for NSCLC subtypes characterized by ERBB2 amplification.
Basic study design
1. Cells are maintained at exponential growth prior to injection.
2. Trypsinized Calu-3 cells are collected and viable cell counts are then determined using MTT assay (required 99% cell viability). The collected cell suspension is then adjusted to the appropriate cell density.
3. The mice (athymic nu/nu or BALB/c; 10-11 weeks old) receive a single subcutaneous injection in the hind leg flank of 200 µL containing one million cells of the Matrigel/Calu-3 cells suspension.
4. All injection sites are palpated up to three times weekly to determine tumor establishment. Tumors are then measured via digital calipers until they reach an average size of 90-140 mm3.
5. Animals are then randomized into predetermined treatment cohorts. Administration of the compound of interest (test article) is performed according to the customer supplied treatment schedule.
6. Mouse weights are recorded 2-3 times weekly and tumors are measured daily.
7. Animals are euthanized when the tumor size reaches 2,000 mm3 or the predetermined tumor size limit per approved IACUC protocol.
8. Necropsy and tissue collection are performed as defined for termination of experiment.
9. Tumors are excised, documented by digital imaging and weighed.
10. A standard gross necropsy is performed and the tissues are collected for further downstream analysis.
11. Tumors/tissues are snap frozen in LN2, stabilized in RNA later reagent, prepared for pathology/histological evaluation, or nucleic acid isolated for downstream genetic analysis.
Get Instant Quote for
Calu-3 Xenograft Model
Animal handling at the Altogen Labs facility is regulated by IACUC and compliant to GLP standards. Following acclimatization, mice are sorted according to body mass. The animals are extensively examined daily for tumor appearance and clinical signs. Detailed experimental procedures, health reports and data provided to the client, including methods, results, discussion and raw data along with statistical analysis. Additional services available include collection of tissue, histology, isolation of total protein or DNA/RNA and analysis of gene expression.
Following options are available for the Calu-3 xenograft model:
- Calu-3 Tumor Growth Delay (TGD; latency)
- Calu-3 Tumor Growth Inhibition (TGI)
- Dosing frequency and duration of dose administration
- Dosing route (intravenous, intratracheal, continuous infusion, intraperitoneal, intratumoral, oral gavage, topical, intramuscular, subcutaneous, intranasal, using cutting-edge micro-injection techniques and pump-controlled IV injection)
- Calu-3 tumor immunohistochemistry
- Alternative cell engraftment sites (orthotopic transplantation, tail vein injection and left ventricular injection for metastasis studies, injection into the mammary fat pad, intraperitoneal injection)
- Cell count and blood chemistry analysis
- Toxicity and survival (optional: performing a broad health observation program)
- Gross necropsies and histopathology
- Positive control group employing cyclophosphamide, at a dosage of 20 mg/kg
