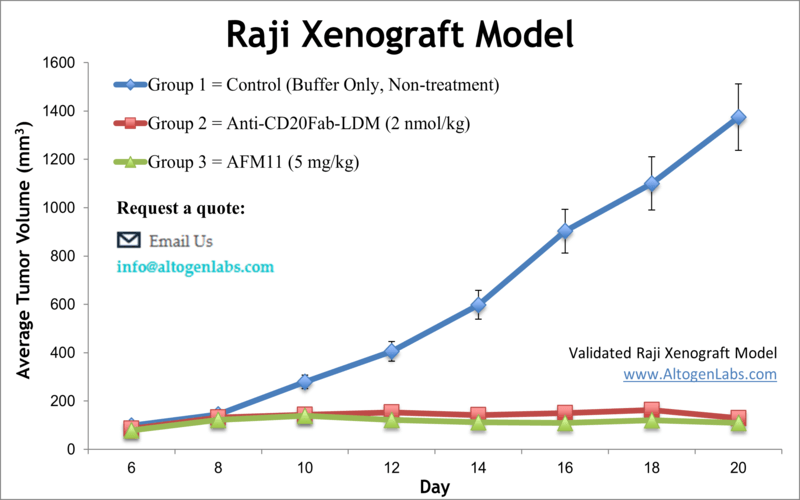
Raji xenograft model
Raji cells are a human B lymphocyte cell line that was established from the blood of a patient with Burkitt lymphoma in 1963. Burkitt lymphoma is a type of non-Hodgkin lymphoma that is characterized by the abnormal growth of B cells. Raji cells have been a valuable tool for immunological research and have provided important insights into the mechanisms of B cell function and the development of B cell-related disorders. Non-Hodgkin lymphoma (NHL) is cancer that starts in lymphocytes and can be diagnosed at any age, comprising approximately 4 percent of all malignancies in the United States. Despite being one of the most frequent pediatric cancers, more than 50 percent of all NHL patients are age 65 or older, as per the American Cancer Society. The lymphoblastoid Raji cell line was isolated from an 11-year-old black male patient with Burkitt’s lymphoma. Raji is the first hematopoietic human cell line and an excellent transfection host for a variety of molecular biology applications. The Raji cell line is resistant to vesicular stomatitis and polio viruses. A 2016 study by Richter et al. published in Molecular Therapy, evaluated safety and efficacy of Ad35K++, using Raji cells as well as Raji tumor xenograft models in vivo. Ad35K++ is a small recombinant protein, isolated from an adenovirus, which binds to CD46. The article indicates that the effect of rituximab treatment significantly improves in combination with Ad35K++ in the Raji xenograft lymphoma model. These findings suggest that Ad35K++ could be used in combination with rituximab for the treatment of NHL patients. A 2002 Clinical Cancer Research article by Michel et al. studied the antibody localization in the Raji B-cell xenograft model of three specific antibodies for CD20, CD147 and MHC class II. Typically the antibodies’ uptake is slow, and the group found that when residualizing radiolabels were used (such as 125I iodo-dilactitol-tyramine and 111I n-benzyl-diethylenetriaminepentaacetic acid) increased Ab catabolism and tumor accumulation. This supports the use of residualizing radiolabels in radioimmunotherapy. Another study that used the Raji B-cell lymphoma model is by Tsukahara et al. (2013), where the group studied chimeric antigen receptors (CARs) specific for CD19 (CD19-CAR) in order to target-engineer T-cells. Results showed that CD19-CAR modified T-cells were able to accumulate at tumor sites and successfully lyse the tumor cells, supporting the use of this technique in adoptive T-cell therapy against refractory B-cell lymphoma. The last example for the use of Raji cells is the 2008 Clinical Cancer Research study by Lapalombella et al. which developed a stable Raji subline that overexpresses CD52 in order to better study CD-52 targeted therapies (ex. Alemtuzumab). There had not been a reliable B-cell line expressing high levels of CD52, a well-known antitumor target, and results demonstrated the successful production of the novel Raji cell line and exemplified its use in the confirmation of increased cytotoxic effects of alemtuzumab in this novel model. The Raji cell line (human lymphoma; Burkitt’s lymphoma) is used to create the CDX (Cell Line Derived Xenograft) Raji xenograft mouse model. The Raji xenograft model facilitates tumor growth inhibition studies by immunotoxins (e.g. 2L-Rap-hLL1-γ4P) and antibody-based therapies (e.g. anti-CD20Fab-LDM, heparanase-neutralizing monoclonal antibodies).
Download Altogen Labs Raji Xenograft Model PowerPoint Presentation: ![]()
Basic study design
- Raji cells are prepared for injection by cell count and viability determination. The Matrigel + Raji cell suspension density is adjusted to 10,000 cells/µL (1.5 x 106 cells per injection).
- NOD/SCID mice (10-12 w.o.) will receive single s.c. injections to the flank of a hind leg. Animal flank injection sites are palpated until tumors are established. Tumors are continually measured until an average size of 60-90 mm3 is reached. Animal randomization into grouping cohorts and test article dosing follows the treatment schedule.
- Tumor measurements and mouse weights are recorded. End of study necropsies begin as tumor size reaches 2,000 mm3 and tissue collections are performed as defined by client.
- Tumors are excised & weighed. Optional digital imaging of the resected tumors is available. Tumors/tissues are stored by snap freezing (LN2), submerged in RNAlater, or fixed in 10% NBF.
Get Instant Quote for
Raji Xenograft Model
Xenograft animal models are used to assess the effectiveness of drugs against specific types of cancer. New medicines are tested on staged tumor growths that have been engrafted via subcutaneous or orthotopic inoculation in an immunocompromised mouse or rat model. All clinically approved anti-cancer agents have been evaluated with conventional nonclinical in vivo models. Xenograft studies can be highly complex, starting with the selection of the appropriate animal model, choice of tumorigenic cell line, administration method, dosing, analysis of tumor growth rates and tumor analysis (histology, mRNA and protein expression levels). Researchers investigating the role of specific proteins or gene products in regulating tumor growth can benefit from development of protein overexpression (genetically engineered to ectopically express proteins, tumor suppressors, or oncogenes) and RNAi cell lines with long term gene silencing. We provide detailed experimental procedures, health reports and data (all-inclusive report is provided to the client that includes methods, results, discussion and raw data along with statistical analysis). Additional services available include collection of tissue, histology, isolation of total protein or RNA and analysis of gene expression.
Following options are available for the Raji xenograft model:
- Raji Tumor Growth Delay (TGD; latency)
- Raji Tumor Growth Inhibition (TGI)
- Dosing frequency and duration of dose administration
- Raji tumor immunohistochemistry
- Blood chemistry analysis
- Toxicity and survival (optional: performing a broad health observation program)
- Gross necropsies and histopathology
- Lipid distribution and metabolic assays
- Imaging studies: Fluorescence-based whole body imaging
