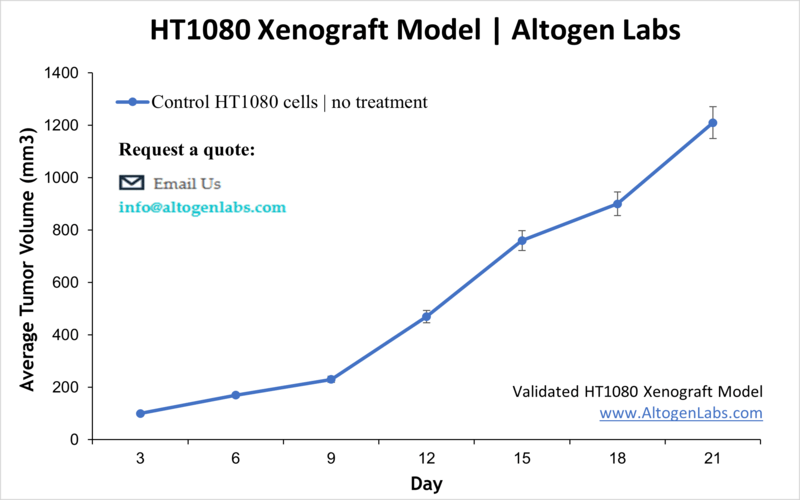
HT1080 Xenograft Model
HT1080 sarcoma cells are commonly used in preclinical studies to evaluate the efficacy and toxicity of new anti-cancer drugs and to study the mechanisms underlying tumor growth and drug resistance. Sarcoma is a subtype of cancer that occurs in connective tissue cells (mesenchymal) that are transformed. Also known as sarcomatas, these cancers can affect cartilage, cancellous bone, blood, fat, vasculature and muscles. These cancers are relatively rare. The HT1080 cell line was established from a 35yo male patient with fibrosarcoma who had not undergone previous therapy (radiotherapy or chemotherapy). The HT1080 model has been used in many biomedical research studies. Misra et al. (PlosOne 2012) used the HT1080 cell line for its ability to form aggressive angiogenic tumors in xenografted mice in order to study hypoxia-induced angiogenesis in tumors. Data showed that during hypoxia, HIF-1α mediates an upregulation of neuropilin-1 (NRP-1) that causes vasculogenic mimicry and tumor mimicry. Hanyu et al. (Cancer Science, 2009) used HT1080 xenografts to study imaging of angiogenesis in vivo. The group used a VEGF antibody to repress angiogenesis and found that the antibody also decreased lung metastasis using in vivo fluorescence to image tumor progression. Lastly, a 2010 Cancer Research article (Casal et al.) used HT1080 cells to study tumor plasticity with endothelial-like properties. Data showed the cells had the ability to form pseudovascular structures and identified the protease ADAMTS1 as critical for cells to display an endothelial-like gene signature. The HT1080 xenograft model has been used to study fibrosarcoma tumor biology.
HT1080 Sarcoma Orthotopic And Metastatic Xenograft Model: Download ![]()
Sarcomas are a diverse group of malignant tumors that arise from mesenchymal tissues, including bone, muscle, fat, and connective tissues. They are relatively rare but often aggressive, with limited treatment options for advanced or metastatic disease. Xenograft models, in which human sarcoma cells or patient-derived tumor tissues are implanted into immunodeficient mice, play a critical role in preclinical research by providing a biologically relevant platform for studying tumor growth, progression, and therapeutic response. Subcutaneous xenografts offer a convenient and reproducible method for evaluating drug efficacy, while orthotopic and patient-derived xenograft (PDX) models better recapitulate the tumor microenvironment and metastatic behavior. Cell-line derived xenografts (CDXs) serve as a foundational tool for testing standardized treatment regimens, providing a reproducible system for evaluating drug responses across multiple experimental conditions. These models are particularly valuable for testing novel targeted therapies, chemotherapeutic agents, and immunotherapies in a controlled setting before clinical trials. Additionally, xenografts enable researchers to investigate mechanisms of tumor resistance, biomarker discovery, and personalized treatment approaches.
HT1080 Cell Line
The HT1080 cell line is a widely used human fibrosarcoma model derived from the connective tissue of a patient with fibrosarcoma. As an epithelial-like adherent cell line, HT1080 cells exhibit rapid proliferation and the ability to form tumors when implanted into immunodeficient mice, making them valuable for cancer research and drug development. These cells express key oncogenic mutations, which contributes to their aggressive phenotype and makes them a useful model for studying tumor invasion, metastasis, and therapeutic responses. HT-1080 cells are commonly used in assays evaluating extracellular matrix degradation, angiogenesis, and cell migration due to their high invasive potential. Additionally, they serve as a platform for testing novel chemotherapeutic agents, targeted therapies, and gene-editing technologies. Their robust and reproducible growth characteristics allow for consistent in vitro and in vivo studies, supporting advancements in fibrosarcoma research. HT1080 cells have been instrumental in understanding the molecular mechanisms underlying sarcoma progression and resistance to treatment.
Fibrosarcoma Research Using Orthotopic HT1080 Xenograft Model
The orthotopic HT1080 model provides a biologically relevant platform for studying fibrosarcoma within its native tissue environment. In this model, HT1080 cells are implanted into anatomically appropriate sites, such as the retroperitoneal space or intramuscular compartments, to better mimic the tumor microenvironment observed in human sarcomas. This approach allows for the investigation of tumor growth, invasion, and interactions with surrounding stromal and vascular components. Compared to subcutaneous models, orthotopic HT1080 xenografts more accurately replicate disease progression, including local invasion and metastatic dissemination. These models are particularly valuable for assessing novel therapeutics that target tumor-microenvironment interactions, angiogenesis, and metastatic pathways. Advanced imaging techniques such as bioluminescence and MRI enable real-time monitoring of tumor development and treatment responses. While technically more challenging than subcutaneous implantation, the orthotopic HT1080 model offers a superior preclinical system for evaluating the efficacy of targeted therapies and improving translational cancer research.
Sarcoma Metastatic HT1080 Model
The metastatic HT1080 model is utilized for studying the mechanisms of fibrosarcoma dissemination and evaluating anti-metastatic therapies. In this model, HT1080 cells are introduced into immunodeficient mice via intravenous, intracardiac, or orthotopic injection, allowing for the development of spontaneous or experimental metastases in distant organs such as the lungs, liver, and lymph nodes. This system closely mimics the metastatic cascade observed in human sarcomas, including intravasation, circulation, extravasation, and colonization at secondary sites. The model is widely used to investigate key regulators of metastasis, such as cell adhesion molecules, extracellular matrix remodeling, and hypoxia-driven pathways. Additionally, it serves as a preclinical platform for testing novel therapeutics, including inhibitors of invasion, angiogenesis, and immune-based strategies. Real-time imaging modalities, such as bioluminescence and fluorescence labeling, enable longitudinal tracking of metastatic progression and treatment response.
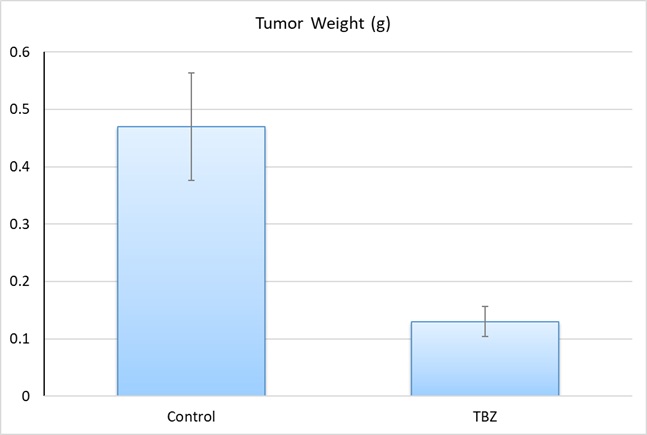
HT1080 Fibrosarcoma Cells Reveal Thiabendazole as a Novel Anti-Cancer Agent
Dr. Cha HJ, et al (PLOS Biology journal) demonstrated that TBZ, an antifungal drug in clinical use for over 40 years, inhibits angiogenesis and disrupts tumor vasculature, making it a promising candidate for cancer therapy. Using HT1080 xenografts in mice, TBZ treatment significantly reduced tumor growth and vascular density without directly affecting tumor cell proliferation in vitro. The study demonstrates that TBZ acts by targeting endothelial cell behavior rather than inducing apoptosis, differentiating it from other VDAs. Mechanistically, TBZ exerts its effects through microtubule disruption and Rho kinase activation, leading to endothelial cell disassembly and inhibition of angiogenesis. Additionally, its FDA approval and established safety profile make TBZ an attractive candidate for rapid clinical translation as an adjunct therapy for highly vascularized tumors.
HT1080 and the Discovery of a Mutant IDH-1 Inhibitor for Cancer Therapy
Another study conducted by Zheng M, et al., published by Oncotarget journal, explores the potential of clomifene, a selective estrogen receptor modulator, as a novel inhibitor of mutant isocitrate dehydrogenase 1 (IDH1), a key driver in certain cancers; including HT1080 fibrosarcoma. The study found that clomifene selectively inhibits mutant IDH1 activity, leading to a dose-dependent reduction in D-2-hydroxyglutarate (D-2HG) production, a known oncometabolite that drives tumor progression. In HT1080 xenograft mouse models, oral administration of clomifene significantly suppressed tumor growth, further supporting its therapeutic potential. Knockdown experiments in HT1080 cells revealed that reducing mutant IDH1 expression decreased the sensitivity to clomifene, confirming that its anti-cancer effects are mutant IDH1-dependent. Additionally, clomifene treatment reversed histone hypermethylation, a hallmark of IDH1-mutant cancers, suggesting epigenetic restoration as a mechanism of action. Given its established safety profile as an FDA-approved drug, clomifene represents a promising candidate for repurposing in IDH1-mutant cancers, potentially accelerating its translation into clinical applications.
Evaluating Fibrosarcoma Therapy Using the HT1080 Subcutaneous Model
The subcutaneous HT1080 model is a widely used preclinical platform for studying fibrosarcoma tumor growth and evaluating therapeutic efficacy. In this model, HT1080 cells are injected subcutaneously, typically into the hind flank of immunodeficient mice, allowing for the development of measurable, localized tumors. This approach provides a highly reproducible system for assessing tumor progression, drug response, and biomarker expression under controlled conditions. Due to its accessibility, the subcutaneous model enables frequent tumor monitoring using caliper measurements and advanced imaging techniques. It is commonly employed in preclinical screening chemotherapeutic agents, targeted therapies, and combination treatments to determine their impact on tumor size and growth kinetics.
HT1080 Fibrosarcoma Cells and Ferroptosis: A Novel Therapeutic Target
HT1080 fibrosarcoma cells serve as a crucial model for studying ferroptosis, a unique form of regulated cell death driven by iron-dependent lipid peroxidation. Recent research has demonstrated that conjugated fatty acids (CFAs), including α-eleostearic acid (ESA), induce ferroptosis in HT1080 cells through the chaperone-mediated autophagy (CMA) degradation of glutathione peroxidase 4 (GPX4). GPX4 is a key enzyme that prevents lipid peroxidation, and its degradation sensitizes HT1080 cells to oxidative stress. The mechanism involves mitochondrial targeting of CFAs, leading to reactive oxygen species (ROS) generation, lipid peroxidation, and ultimately, ferroptosis cell death. This pathway is dependent on LAMP2A, a key regulator of CMA, as its knockout confers resistance to CFA-induced ferroptosis. In vivo studies using HT1080 xenografts further confirm that oral administration of ESA-rich oils suppresses tumor growth through increased lipid peroxidation and GPX4 degradation. These findings highlight the vulnerability of HT1080 fibrosarcoma cells to ferroptosis and suggest CFA-based ferroptosis induction as a potential therapeutic approach for targeting fibrosarcoma and other aggressive cancers.
Basic Study Design
- HT1080 cells are maintained in exponential growth phase under aseptic conditions.
- Cells are trypsinized and cell count viability is determined using a trypan blue exclusion assay (97-98% of cell viability is required). HT1080 cell suspension is adjusted to appropriate density.
- Each mouse is singly subcutaneously injected into the right flank with 106 cells in 150-200 µL of a Matrigel-HT1080 cell suspension.
- The injection sites are palpated up to three times weekly until tumors are established to an average size of 50-150 mm3 as measured via digital calipers.
- Animals are randomized into treatment groups. Administration of test compound is performed according to the pre-established treatment schedule.
- Mice weights are measured and recorded 2-3 times weekly; tumors are measured and recorded daily.
- End of study is reached when tumor size reaches 2,000 mm3 or the predetermined size limit per approved IACUC protocol.
- Final necropsy and tissue collections are performed for appropriate downstream analysis. Tumors are excised, weighed and documented by digital imaging. Tumors and tissues can be stabilized in RNAlater, snap frozen in LN2 or prepared for histology.
HT1080 Sarcoma Orthotopic And Metastatic Xenograft Model: Download ![]()
