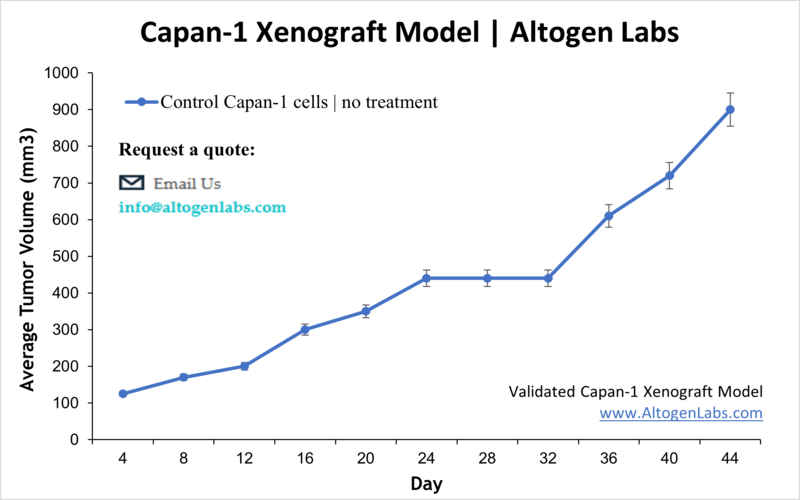
Capan-1 Xenograft Model
The pancreas is normally responsible for secreting digestion-aiding enzymes and sugar metabolism-regulating hormones. Pancreatic cancer generally has no early-stage symptoms; it is often detected in later stages and therefore has a poor prognosis. Surgery, radiation, and chemotherapy are all treatment options for pancreatic cancer. Risk factors of pancreatic cancer include smoking tobacco, diabetes, genetics and obesity. The Capan-1 cell line was taken from a metastatic site of a 40 year old patient with pancreatic cancer. The Capan-1 model has been used in many pancreatic cancer research studies. A 2010 Cancer Science study (Komoto et al.) used the Capan-1 cell line in an investigation into the combination of lapatinib, a dual HER2 and EGFR inhibitor, with S1, a fluoropyrimidine derivative. Results demonstrated xenograft tumor growth suppression with lapatinib alone and in combination with S-1; tumors with a higher HER2 expression were more sensitive to lapatinib. Qu et al. (Cancer Biology & Therapy, 2005) used the Capan-1 model in the study of targeted alpha therapy; the group used C595 antibodies labeled with 213Bi using CHX.A as a chelator (213Bi-CHX.A-C595). Data showed in vitro tumor regression and in vivo tumor growth inhibition; this was specifically targeted against MUC-1 overexpressing tumors. Finally, Ansari et al. (Anticancer Research, 2014) published a study analyzing the histology and expression of MUC4, a surface glycoprotein, in pancreatic cancer. The group used the Capan-1, CD18/HPAF and HPAF-II cell lines and found that Capan-1 tumors had the highest alpha-smooth muscle actin (a measure of cancer-associated fibroblasts) and collagen levels. This suggests that Capan-1 is a suitable model for studying MUC4 targeted treatments. The Capan-1 cell line is used to create the CDX (Cell Line Derived Xenograft) Capan-1 xenograft mouse model. The Capan-1 xenograft model has been used to study pancreatic cancer treatments (lapatinib, S-1, small molecule inhibitors, immunotherapies, etc.).
Capan1 xenografts are commonly used in preclinical studies to evaluate the efficacy of potential therapies for pancreatic cancer. Researchers can test the effects of chemotherapy drugs, targeted therapies, and other treatments on the growth and progression of Capan1 tumors in NOD/SCID immonocompromised mice. By studying the response of Capan1 xenografts to different treatments, researchers gain insights into the mechanisms underlying pancreatic cancer and identify new therapeutic targets.
Basic Study Design
- Capan-1 cells are maintained in exponential growth phase under aseptic conditions.
- Cells are trypsinized and cell count viability is determined using a trypan blue exclusion assay (98% of cell viability is required). Capan-1 cell suspension is adjusted to appropriate density.
- Each mouse is singly subcutaneously injected into the right flank with 106 cells in 150-200 µL of a Matrigel-Capan1 cell suspension.
- The injection sites are palpated up to three times weekly until tumors are established to an average size of 50-150 mm3 as measured via digital calipers.
- Animals are randomized into treatment groups. Administration of test compound is performed according to the pre-established treatment schedule.
- Mice weights are measured and recorded 3 times weekly; tumors are measured and recorded daily.
- End of study is reached when tumor size reaches 2,000 mm3 or the predetermined size limit per approved IACUC protocol.
- Final necropsy and tissue collections are performed for appropriate downstream analysis. Tumors are excised, weighed and documented by digital imaging. Tumors and tissues can be stabilized in RNAlater, snap frozen in LN2 or prepared for histology.
